Differential Inhibition of Human Atherosclerotic Plaque-Induced Platelet Activation by Dimeric GPVI-Fc and Anti-GPVI Antibodies: Functional and Imaging Studies
- PMID: 26046734
- PMCID: PMC4452546
- DOI: 10.1016/j.jacc.2015.03.573
Differential Inhibition of Human Atherosclerotic Plaque-Induced Platelet Activation by Dimeric GPVI-Fc and Anti-GPVI Antibodies: Functional and Imaging Studies
Abstract
Background: Glycoprotein VI (GPVI) is the essential platelet collagen receptor in atherothrombosis, but its inhibition causes only a mild bleeding tendency. Thus, targeting this receptor has selective antithrombotic potential.
Objectives: This study sought to compare compounds interfering with platelet GPVI-atherosclerotic plaque interaction to improve current antiatherothrombotic therapy.
Methods: Human atherosclerotic plaque-induced platelet aggregation was measured in anticoagulated blood under static and arterial flow conditions (550/s, 1,100/s, and 1,500/s). Inhibition by dimeric GPVI fragment crystallizable region of IgG (Fc) masking GPVI binding sites on collagen was compared with that of 3 anti-GPVI antibodies: BLO8-1, a human domain antibody; 5C4, a fragment antigen-binding (Fab fragment) of monoclonal rat immunoglobulin G; and m-Fab-F, a human recombinant sFab against GPVI dimers.
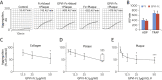
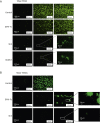
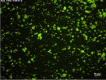
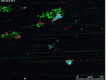
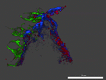
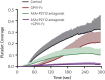
Results: GPVI-Fc reduced plaque-triggered platelet aggregation in static blood by 51%, BLO8-1 by 88%, and 5C4 by 93%. Under arterial flow conditions, BLO8-1 and 5C4 almost completely inhibited platelet aggregation while preserving platelet adhesion on plaque. Inhibition by GPVI-Fc, even at high concentrations, was less marked but increased with shear rate. Advanced optical imaging revealed rapid persistent GPVI-Fc binding to collagen under low and high shear flow, upstream and downstream of plaque fragments. At low shear particularly, platelets adhered in plaque flow niches to GPVI-Fc-free segments of collagen fibers and recruited other platelets onto aggregates via ADP and TxA2 release.
Conclusions: Anti-GPVI antibodies inhibit atherosclerotic plaque-induced platelet aggregation under static and flow conditions more effectively than GPVI-Fc. However, potent platelet inhibition by GPVI-Fc at a higher shear rate (1,500/s) suggests localized antithrombotic efficacy at denuded or fissured stenotic high-risk lesions without systemic bleeding. The compound-specific differences have relevance for clinical trials targeting GPVI-collagen interaction combined with established antiplatelet therapies in patients with spontaneous plaque rupture or intervention-associated plaque injury.
Keywords: antithrombotic; atherothrombosis; glycoprotein VI; plaque rupture.
Copyright © 2015 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures










References
-
- Fuster V., Moreno P.R., Fayad Z.A., Corti R., Badimon J.J. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol. 2005;46:937–954. - PubMed
-
- Badimon L., Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Int Med. 2014;276:618–632. - PubMed
-
- Reininger A.J., Bernlochner I., Penz S.M., et al. A 2-step mechanism of arterial thrombus formation induced by human atherosclerotic plaques. J Am Coll Cardiol. 2010;55:1147–1158. - PubMed
-
- Penz S., Reininger A.J., Brandl R., et al. Human atheromatous plaques stimulate thrombus formation by activating platelet glycoprotein VI. FASEB J. 2005;19:898–909. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

