Quantitative Analyses of Force-Induced Amyloid Formation in Candida albicans Als5p: Activation by Standard Laboratory Procedures
- PMID: 26047318
- PMCID: PMC4457901
- DOI: 10.1371/journal.pone.0129152
Quantitative Analyses of Force-Induced Amyloid Formation in Candida albicans Als5p: Activation by Standard Laboratory Procedures
Abstract
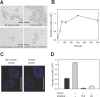
Candida albicans adhesins have amyloid-forming sequences. In Als5p, these amyloid sequences cluster cell surface adhesins to create high avidity surface adhesion nanodomains. Such nanodomains form after force is applied to the cell surface by atomic force microscopy or laminar flow. Here we report centrifuging and resuspending S. cerevisiae cells expressing Als5p led to 1.7-fold increase in initial rate of adhesion to ligand coated beads. Furthermore, mechanical stress from vortex-mixing of Als5p cells or C. albicans cells also induced additional formation of amyloid nanodomains and consequent activation of adhesion. Vortex-mixing for 60 seconds increased the initial rate of adhesion 1.6-fold. The effects of vortex-mixing were replicated in heat-killed cells as well. Activation was accompanied by increases in thioflavin T cell surface fluorescence measured by flow cytometry or by confocal microscopy. There was no adhesion activation in cells expressing amyloid-impaired Als5pV326N or in cells incubated with inhibitory concentrations of anti-amyloid dyes. Together these results demonstrated the activation of cell surface amyloid nanodomains in yeast expressing Als adhesins, and further delineate the forces that can activate adhesion in vivo. Consequently there is quantitative support for the hypothesis that amyloid forming adhesins act as both force sensors and effectors.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

