Phenotypic diversity within a Pseudomonas aeruginosa population infecting an adult with cystic fibrosis
- PMID: 26047320
- PMCID: PMC4456944
- DOI: 10.1038/srep10932
Phenotypic diversity within a Pseudomonas aeruginosa population infecting an adult with cystic fibrosis
Abstract
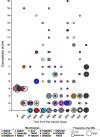
Chronic airway infections caused by Pseudomonas aeruginosa contribute to the progression of pulmonary disease in individuals with cystic fibrosis (CF). In the setting of CF, within-patient adaptation of a P. aeruginosa strain generates phenotypic diversity that can complicate microbiological analysis of patient samples. We investigated within- and between- sample diversity of 34 phenotypes among 235 P. aeruginosa isolates cultured from sputum samples collected from a single CF patient over the span of one year, and assessed colony morphology as a screening tool for predicting phenotypes, including antimicrobial susceptibilities. We identified 15 distinct colony morphotypes that varied significantly in abundance both within and between sputum samples. Substantial within sample phenotypic heterogeneity was also noted in other phenotypes, with morphotypes being unreliable predictors of antimicrobial susceptibility and other phenotypes. Emergence of isolates with reduced susceptibility to β-lactams was observed during periods of clinical therapy with aztreonam. Our findings confirm that the P. aeruginosa population in chronic CF lung infections is highly dynamic, and that intra-sample phenotypic diversity is underestimated if only one or few colonies are analyzed per sample.
Figures



References
-
- Schaedel C. et al. Predictors of deterioration of lung function in cystic fibrosis. Pediatr. Pulmonol. 33, 483–491 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

