Predictive Low-Glucose Insulin Suspension Reduces Duration of Nocturnal Hypoglycemia in Children Without Increasing Ketosis
- PMID: 26049549
- PMCID: PMC4477332
- DOI: 10.2337/dc14-3053
Predictive Low-Glucose Insulin Suspension Reduces Duration of Nocturnal Hypoglycemia in Children Without Increasing Ketosis
Erratum in
-
Erratum. Predictive Low-Glucose Insulin Suspension Reduces Duration of Nocturnal Hypoglycemia in Children Without Increasing Ketosis. Diabetes Care 2015;38:1197-1204.Diabetes Care. 2015 Sep;38(9):1813. doi: 10.2337/dc15-er09. Diabetes Care. 2015. PMID: 26294776 Free PMC article. No abstract available.
Abstract
Objective: Nocturnal hypoglycemia can cause seizures and is a major impediment to tight glycemic control, especially in young children with type 1 diabetes. We conducted an in-home randomized trial to assess the efficacy and safety of a continuous glucose monitor-based overnight predictive low-glucose suspend (PLGS) system.
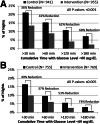
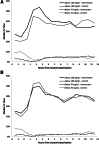
Research design and methods: In two age-groups of children with type 1 diabetes (11-14 and 4-10 years of age), a 42-night trial for each child was conducted wherein each night was assigned randomly to either having the PLGS system active (intervention night) or inactive (control night). The primary outcome was percent time <70 mg/dL overnight.
Results: Median time at <70 mg/dL was reduced by 54% from 10.1% on control nights to 4.6% on intervention nights (P < 0.001) in 11-14-year-olds (n = 45) and by 50% from 6.2% to 3.1% (P < 0.001) in 4-10-year-olds (n = 36). Mean overnight glucose was lower on control versus intervention nights in both age-groups (144 ± 18 vs. 152 ± 19 mg/dL [P < 0.001] and 153 ± 14 vs. 160 ± 16 mg/dL [P = 0.004], respectively). Mean morning blood glucose was 159 ± 29 vs. 176 ± 28 mg/dL (P < 0.001) in the 11-14-year-olds and 154 ± 25 vs. 158 ± 22 mg/dL (P = 0.11) in the 4-10-year-olds, respectively. No differences were found between intervention and control in either age-group in morning blood ketosis.
Conclusions: In 4-14-year-olds, use of a nocturnal PLGS system can substantially reduce overnight hypoglycemia without an increase in morning ketosis, although overnight mean glucose is slightly higher.
Trial registration: ClinicalTrials.gov NCT01823341.
© 2015 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures

References
-
- The DCCT Research Group . Epidemiology of severe hypoglycemia in the diabetes control and complications trial. Am J Med 1991;90:450–459 - PubMed
-
- Davis EA, Keating B, Byrne GC, Russell M, Jones TW. Hypoglycemia: incidence and clinical predictors in a large population-based sample of children and adolescents with IDDM. Diabetes Care 1997;20:22–25 - PubMed
-
- Sovik O, Thordarson H. Dead-in-bed syndrome in young diabetic patients. Diabetes Care 1999;22(Suppl. 2):B40–B42 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

