V0162 a new long-acting bronchodilator for treatment of chronic obstructive lung diseases: preclinical and clinical results
- PMID: 26050967
- PMCID: PMC4462001
- DOI: 10.1186/s12931-015-0227-1
V0162 a new long-acting bronchodilator for treatment of chronic obstructive lung diseases: preclinical and clinical results
Abstract
Background: Long acting bronchodilators are the standard of care in the management of chronic obstructive pulmonary disease (COPD). The aim of this study was to investigate the efficacy and safety of V0162, a novel anticholinergic agent with bronchodilator properties, in preclinical models and in patients with COPD.
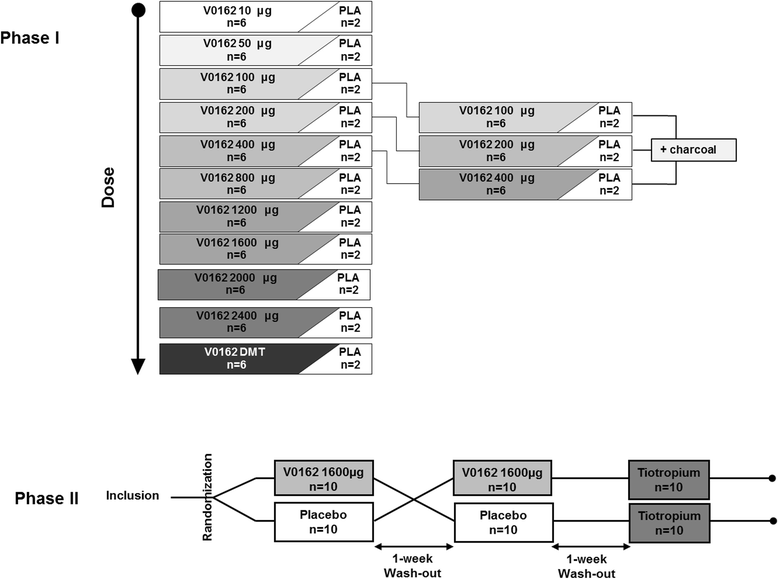
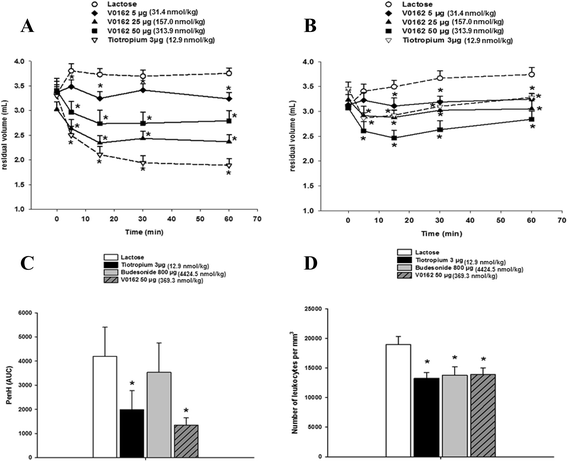
Methods: Guinea pigs were used to evaluate the impact of V0162 on the acetylcholine or histamine-induced bronchoconstriction. V0162 was also investigated in an allergic asthma model on ovalbumin-sensitized guinea pig. For clinical investigations, healthy volunteers were included in a dose-escalation, randomized, placebo-controlled phase I study to determine the maximal tolerated dose, followed by a randomized, placebo-controlled, cross-over phase II study in patients with COPD. V0162 was given via inhalation route. The objectives of the phase I/II study were to assess the safety and efficacy of V0162, in terms of bronchodilation and reduction in hyperinflation.
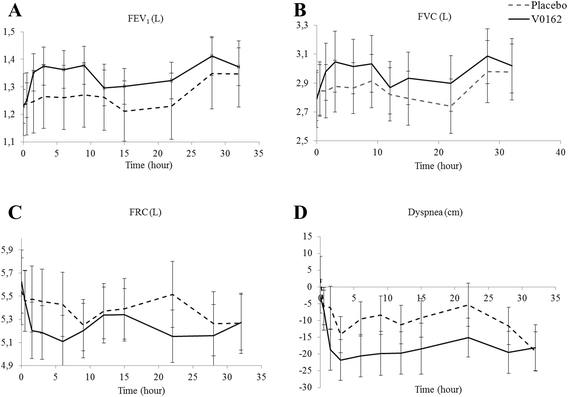
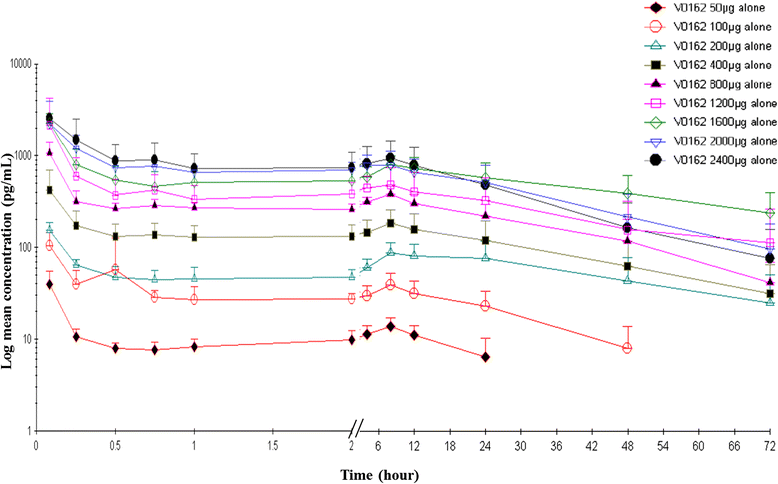
Results: Preclinical results showed that V0162 was able to prevent bronchoconstriction induced either by acetylcholine or histamine. V0162 reversed the bronchoconstriction and airway inflammation caused by ovalbumin challenge in sensitized guinea pigs. In the healthy volunteers study, 88 subjects were enrolled: 66 received V0162 and 22 received placebo. No particular safety concerns were raised. The maximal tolerated dose was not reached and the dose escalation was stopped at 2400 μg. A total of 20 patients with COPD were then enrolled. All patients received a single-dose of V0162 1600 μg and of placebo in two alternating periods. In COPD patients, V0162 demonstrated a significant increase in FEV1 compared with placebo (148 ± 137 ml vs. 36 ± 151 ml, p = 0.003). This bronchodilatory effect was corroborated by a reduction in hyperinflation. There was a trend toward dyspnea relief (change in visual analog scale at 22 h, -15.1 ± 26.0 mm vs.- 5.3 ± 28.8 mm with placebo, p = 0.054). No serious adverse events (AEs) were reported. Most common AEs were productive and non-productive cough, dyspnea and pruritus.
Conclusions: V0162 improved pulmonary function and tended to improve dyspnea in patients with COPD over more than 24 h. The slight plasmatic exposure observed might support the good safety profile.
Trial registration: ClinicalTrials.gov identifier: NCT01348555.
Figures
References
-
- Global strategy for the diagnosis, management, and prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2015. Available from: http://www.goldcopd.org/ - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

