Sequence-engineered mRNA Without Chemical Nucleoside Modifications Enables an Effective Protein Therapy in Large Animals
- PMID: 26050989
- PMCID: PMC4817881
- DOI: 10.1038/mt.2015.103
Sequence-engineered mRNA Without Chemical Nucleoside Modifications Enables an Effective Protein Therapy in Large Animals
Abstract
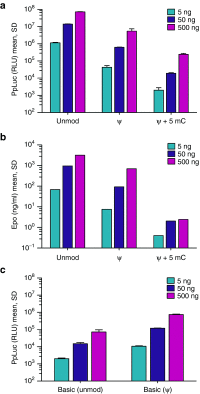
Being a transient carrier of genetic information, mRNA could be a versatile, flexible, and safe means for protein therapies. While recent findings highlight the enormous therapeutic potential of mRNA, evidence that mRNA-based protein therapies are feasible beyond small animals such as mice is still lacking. Previous studies imply that mRNA therapeutics require chemical nucleoside modifications to obtain sufficient protein expression and avoid activation of the innate immune system. Here we show that chemically unmodified mRNA can achieve those goals as well by applying sequence-engineered molecules. Using erythropoietin (EPO) driven production of red blood cells as the biological model, engineered Epo mRNA elicited meaningful physiological responses from mice to nonhuman primates. Even in pigs of about 20 kg in weight, a single adequate dose of engineered mRNA encapsulated in lipid nanoparticles (LNPs) induced high systemic Epo levels and strong physiological effects. Our results demonstrate that sequence-engineered mRNA has the potential to revolutionize human protein therapies.
Figures



Comment in
-
mRNA: Fulfilling the Promise of Gene Therapy.Mol Ther. 2015 Sep;23(9):1416-7. doi: 10.1038/mt.2015.138. Mol Ther. 2015. PMID: 26321183 Free PMC article. No abstract available.
References
-
- Gurdon, JB, Lane, CD, Woodland, HR and Marbaix, G (1971). Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature 233: 177–182. - PubMed
-
- Wolff, JA, Malone, RW, Williams, P, Chong, W, Acsadi, G, Jani, A et al. (1990). Direct gene transfer into mouse muscle in vivo. Science 247(4949 Pt 1): 1465–1468. - PubMed
-
- Conry, RM, LoBuglio, AF, Wright, M, Sumerel, L, Pike, MJ, Johanning, F et al. (1995). Characterization of a messenger RNA polynucleotide vaccine vector. Cancer Res 55: 1397–1400. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

