Inhibition of Nav1.7 channels by methyl eugenol as a mechanism underlying its antinociceptive and anesthetic actions
- PMID: 26051112
- PMCID: PMC4648112
- DOI: 10.1038/aps.2015.26
Inhibition of Nav1.7 channels by methyl eugenol as a mechanism underlying its antinociceptive and anesthetic actions
Abstract
Aim: Methyl eugenol is a major active component extracted from the Chinese herb Asari Radix et Rhizoma, which has been used to treat toothache and other pain. Previous in vivo studies have shown that methyl eugenol has anesthetic and antinociceptive effects. The aim of this study was to determine the possible mechanism underlying its effect on nervous system disorders.
Methods: The direct interaction of methyl eugenol with Na(+) channels was explored and characterized using electrophysiological recordings from Nav1.7-transfected CHO cells.
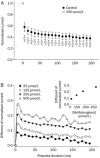
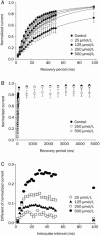
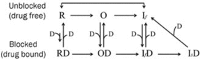
Results: In whole-cell patch clamp mode, methyl eugenol tonically inhibited peripheral nerve Nav1.7 currents in a concentration- and voltage-dependent manner, with an IC50 of 295 μmol/L at a -100 mV holding potential. Functionally, methyl eugenol preferentially bound to Nav1.7 channels in the inactivated and/or open state, with weaker binding to channels in the resting state. Thus, in the presence of methyl eugenol, Nav1.7 channels exhibited reduced availability for activation in a steady-state inactivation protocol, strong use-dependent inhibition, enhanced binding kinetics, and slow recovery from inactivation compared to untreated channels. An estimation of the affinity of methyl eugenol for the resting and inactivated states of the channel also demonstrated that methyl eugenol preferentially binds to inactivated channels, with a 6.4 times greater affinity compared to channels in the resting state. The failure of inactivated channels to completely recover to control levels at higher concentrations of methyl eugenol implies that the drug may drive more drug-bound, fast-inactivated channels into drug-bound, slow-inactivated channels.
Conclusion: Methyl eugenol is a potential candidate as an effective local anesthetic and analgesic. The antinociceptive and anesthetic effects of methyl eugenol result from the inhibitory action of methyl eugenol on peripheral Na(+) channels.
Figures



References
-
- Chinese Pharmacopoeia Committee. Pharmacopoeia of the People's Republic of China. Vol I. Beijing: Chemical Industry Press; 2005. p 159.
-
- Li YL, Tian M, Yu J, Shang MY, Cai SQ. Studies on morphology and aristolochic acid analogue constituents of Asarum campaniflorum and a comparison with two official species of Asari Radix et Rhizoma. J Nat Med 2010; 64: 442–51. - PubMed
-
- Wang D, Wang X, Xia X. Analysis of season variation of methyleugenol and safrole in Asarum heterotropoides by gas chromatography. Se Pu 1997; 15: 85–6. - PubMed
-
- De Vincenzi M, Silano M, Stacchini P, Scazzocchio B. Constituents of aromatic plants: I. Methyleugenol. Fitoterapia 2000; 71: 216–21. - PubMed
-
- Carlini EA, Dallmeier K, Zelger JL. Methyleugenol as a surgical anesthetic in rodents. Experientia 1981; 37: 588–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

