HLTF's Ancient HIRAN Domain Binds 3' DNA Ends to Drive Replication Fork Reversal
- PMID: 26051180
- PMCID: PMC4475461
- DOI: 10.1016/j.molcel.2015.05.013
HLTF's Ancient HIRAN Domain Binds 3' DNA Ends to Drive Replication Fork Reversal
Abstract
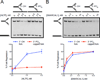
Stalled replication forks are a critical problem for the cell because they can lead to complex genome rearrangements that underlie cell death and disease. Processes such as DNA damage tolerance and replication fork reversal protect stalled forks from these events. A central mediator of these DNA damage responses in humans is the Rad5-related DNA translocase, HLTF. Here, we present biochemical and structural evidence that the HIRAN domain, an ancient and conserved domain found in HLTF and other DNA processing proteins, is a modified oligonucleotide/oligosaccharide (OB) fold that binds to 3' ssDNA ends. We demonstrate that the HIRAN domain promotes HLTF-dependent fork reversal in vitro through its interaction with 3' ssDNA ends found at forks. Finally, we show that HLTF restrains replication fork progression in cells in a HIRAN-dependent manner. These findings establish a mechanism of HLTF-mediated fork reversal and provide insight into the requirement for distinct fork remodeling activities in the cell.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


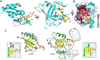



Comment in
-
Bending Forks and Wagging Dogs--It's about the DNA 3' Tail.Mol Cell. 2015 Jun 18;58(6):972-3. doi: 10.1016/j.molcel.2015.06.005. Mol Cell. 2015. PMID: 26091346 Free PMC article.
References
-
- Alabert C, Bukowski-Wills J-C, Lee S-B, Kustatscher G, Nakamura K, de Lima Alves F, Menard P, Mejlvang J, Rappsilber J, Groth A. Nascent chromatin capture proteomics determines chromatin dynamics during DNA replication and identifies unknown fork components. Nat. Cell Biol. 2014;16:281–293. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

