Motor Learning Consolidates Arc-Expressing Neuronal Ensembles in Secondary Motor Cortex
- PMID: 26051420
- PMCID: PMC4474764
- DOI: 10.1016/j.neuron.2015.05.022
Motor Learning Consolidates Arc-Expressing Neuronal Ensembles in Secondary Motor Cortex
Abstract
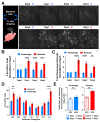
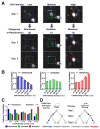
Motor behaviors recruit task-specific neuronal ensembles in motor cortices, which are consolidated over subsequent learning. However, little is known about the molecules that can identify the participating neurons and predict the outcomes of the consolidation process. Using a mouse rotarod-learning task, we showed that lesion or inactivation of the secondary motor (M2) cortex disrupts learning of skilled movements. We tracked the endogenous promoter activity of the neuronal activity-regulated gene Arc in individual M2 neurons during rotarod learning by in vivo two-photon imaging of a knockin reporter. We found that task training initially recruits Arc-promoter-activated neurons and then consolidates them into a specific ensemble exhibiting persistent reactivation of Arc-promoter. The intensity of a neuron's initial Arc-promoter activation predicts its reactivation probability and neurons with weak initial Arc-promoter activation are dismissed from the ensemble during subsequent training. Our findings demonstrate a task-specific Arc-dependent cellular consolidation process in M2 cortex during motor learning.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


References
-
- Brecht M. Movement, confusion, and orienting in frontal cortices. Neuron. 2011;72:193–196. - PubMed
-
- Buitrago MM, Schulz JB, Dichgans J, Luft AR. Short and long-term motor skill learning in an accelerated rotarod training paradigm. Neurobiol Learn Mem. 2004;81:211–216. - PubMed
-
- Costa RM, Cohen D, Nicolelis MA. Differential corticostriatal plasticity during fast and slow motor skill learning in mice. Curr Biol. 2004;14:1124–1134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

