ASXL2 Regulates Glucose, Lipid, and Skeletal Homeostasis
- PMID: 26051940
- PMCID: PMC4472564
- DOI: 10.1016/j.celrep.2015.05.019
ASXL2 Regulates Glucose, Lipid, and Skeletal Homeostasis
Abstract
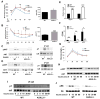
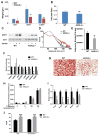
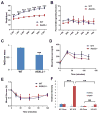
ASXL2 is an ETP family protein that interacts with PPARγ. We find that ASXL2-/- mice are insulin resistant, lipodystrophic, and fail to respond to a high-fat diet. Consistent with genetic variation at the ASXL2 locus and human bone mineral density, ASXL2-/- mice are also severely osteopetrotic because of failed osteoclast differentiation attended by normal bone formation. ASXL2 regulates the osteoclast via two distinct signaling pathways. It induces osteoclast formation in a PPARγ/c-Fos-dependent manner and is required for RANK ligand- and thiazolidinedione-induced bone resorption independent of PGC-1β. ASXL2 also promotes osteoclast mitochondrial biogenesis in a process mediated by PGC-1β but independent of c-Fos. Thus, ASXL2 is a master regulator of skeletal, lipid, and glucose homeostasis.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
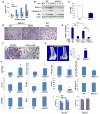
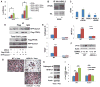
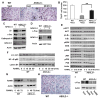
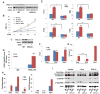
Comment in
-
ASXL2--director of skeletal and metabolic homeostasis.Nat Rev Endocrinol. 2015 Aug;11(8):445. doi: 10.1038/nrendo.2015.102. Epub 2015 Jun 23. Nat Rev Endocrinol. 2015. PMID: 26100099 No abstract available.
References
-
- Bai S, Kopan R, Zou W, Hilton MJ, Ong CT, Long F, Ross FP, Teitelbaum SL. NOTCH1 regulates osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblast lineage cells. J Biol Chem. 2008;283:6509–6518. - PubMed
-
- Chen Z, Vigueira PA, Chambers KT, Hall AM, Mitra MS, Qi N, McDonald WG, Colca JR, Kletzien RF, Finck BN. Insulin resistance and metabolic derangements in obese mice are ameliorated by a novel peroxisome proliferator-activated receptor gamma-sparing thiazolidinedione. J Biol Chem. 2012;287:23537–23548. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AR046523/AR/NIAMS NIH HHS/United States
- DK076729/DK/NIDDK NIH HHS/United States
- 5R01AR03278828/AR/NIAMS NIH HHS/United States
- DK20579/DK/NIDDK NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- R01 AR050211/AR/NIAMS NIH HHS/United States
- P30DK056341/DK/NIDDK NIH HHS/United States
- 5R37AR04652315/AR/NIAMS NIH HHS/United States
- P30AR057235/AR/NIAMS NIH HHS/United States
- 5R01AR05703705/AR/NIAMS NIH HHS/United States
- R01 DK076729/DK/NIDDK NIH HHS/United States
- R01 AR032788/AR/NIAMS NIH HHS/United States
- P60 DK020579/DK/NIDDK NIH HHS/United States
- R01 AR057037/AR/NIAMS NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
- P30 AR057235/AR/NIAMS NIH HHS/United States
- 5R01AR050211/AR/NIAMS NIH HHS/United States
- DK56341/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

