Evaluation of Dalfampridine Extended Release 5 and 10 mg in Multiple Sclerosis: A Randomized Controlled Trial
- PMID: 26052259
- PMCID: PMC4455866
- DOI: 10.7224/1537-2073.2014-040
Evaluation of Dalfampridine Extended Release 5 and 10 mg in Multiple Sclerosis: A Randomized Controlled Trial
Abstract
Background: Dalfampridine extended-release (ER) tablets, 10 mg twice daily, have been shown to improve walking in people with multiple sclerosis. We evaluated the safety and efficacy of dalfampridine-ER 5 mg compared with 10 mg.
Methods: Patients were randomized to double-blind treatment with twice-daily dalfampridine-ER tablets, 5 mg (n = 144) or 10 mg (n = 143), or placebo (n = 143) for 4 weeks. Primary efficacy endpoint was change from baseline walking speed by the Timed 25-Foot Walk 3 to 4 hours after the last dose. At 40% of sites, 2-week change from baseline walking distance was measured by the 6-Minute Walk test.
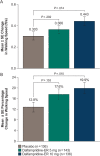
Results: At 4 weeks, walking speed changes from baseline were 0.363, 0.423, and 0.478 ft/s (placebo, dalfampridine-ER 5 mg, and dalfampridine-ER 10 mg, respectively [P = NS]). Post hoc analysis of average changes between pretreatment and on-treatment showed that relative to placebo, only dalfampridine-ER 10 mg demonstrated a significant increase in walking speed (mean ± SE): 0.443 ± 0.042 ft/s versus 0.303 ± 0.038 ft/s (P = .014). Improvement in 6-Minute Walk distance was significantly greater with dalfampridine-ER 10 mg (128.6 ft, P = .014) but not with 5 mg (76.8 ft, P = .308) relative to placebo (41.7 ft). Adverse events were consistent with previous studies. No seizures were reported.
Conclusions: Dalfampridine-ER 5 and 10 mg twice daily did not demonstrate efficacy on the planned endpoint. Post hoc analyses demonstrated significant increases in walking speed relative to placebo with dalfampridine-ER 10 mg. No new safety signals were observed.
Figures





References
-
- Goodman AD, Brown TR, Krupp L et al. Sustained release of oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. Lancet. 2009;373:732–738. - PubMed
-
- Goodman AD, Brown TR, Edwards KR et al. A phase 3 trial of extended release oral dalfampridine in multiple sclerosis. Ann Neurol. 2010;68:494–502. - PubMed
-
- Haut SR, Bienen EJ, Miller A. Clinical overview of the seizure risk of dalfampridine. Expert Opin Drug Saf. 2012;11:651–657. - PubMed
-
- Jara M, Adera M, Adedeji A, Henney HR, III, Carrazana EJ. Dalfampridine extended release tablets: safety profile after 2 years of postmarketing experience in the United States. Poster presented at: 28th Congress of the European Committee for Treatment and Research in Multiple Sclerosis; October 12. 2012; Lyon, France.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
