Hepatitis C virus: Virology, diagnosis and treatment
- PMID: 26052383
- PMCID: PMC4450201
- DOI: 10.4254/wjh.v7.i10.1377
Hepatitis C virus: Virology, diagnosis and treatment
Abstract
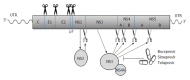
More than twenty years of study has provided a better understanding of hepatitis C virus (HCV) life cycle, including the general properties of viral RNA and proteins. This effort facilitates the development of sensitive diagnostic tools and effective antiviral treatments. At present, serologic screening test is recommended to perform on individuals in the high risk groups and nucleic acid tests are recommended to confirm the active HCV infections. Quantization and genotyping of HCV RNAs are important to determine the optimal duration of anti-viral therapy and predict the likelihood of response. In the early 2000s, pegylated interferon plus ribavirin became the standard anti-HCV treatment. However, this therapy is not ideal. To 2014, boceprevir, telaprevir, simeprevir, sofosbuvir and Harvoni are approved by Food and Drug Administration for the treat of HCV infections. It is likely that the new all-oral, interferon-free, pan-genotyping anti-HCV therapy will be available within the next few years. Majority of HCV infections will be cured by these anti-viral treatments. However, not all patients are expected to be cured due to viral resistance and the high cost of antiviral treatments. Thus, an efficient prophylactic vaccine will be the next challenge in the fight against HCV infection.
Keywords: Diagnosis; Direct acting antivirals; Enzyme immunoassay; Hepatitis C virus; Hepatocellular carcinoma; Host-targeted agents; Interferon; Nucleic acid test; Sofosbuvir; Treatment.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

