Novel roles for protein disulphide isomerase in disease states: a double edged sword?
- PMID: 26052512
- PMCID: PMC4439577
- DOI: 10.3389/fcell.2015.00030
Novel roles for protein disulphide isomerase in disease states: a double edged sword?
Abstract
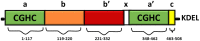
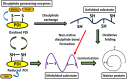
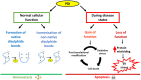
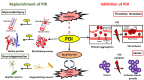
Protein disulphide isomerase (PDI) is a multifunctional redox chaperone of the endoplasmic reticulum (ER). Since it was first discovered 40 years ago the functions ascribed to PDI have evolved significantly and recent studies have recognized its distinct functions, with adverse as well as protective effects in disease. Furthermore, post translational modifications of PDI abrogate its normal functional roles in specific disease states. This review focusses on recent studies that have identified novel functions for PDI relevant to specific diseases.
Keywords: amyotrophic lateral sclerosis; cancer; neurodegnerative diseases; post-translational modifications; protein chaperones; protein disulfide isomerase family.
Figures
References
-
- Ataman-Onal Y., Beaulieu C., Busseret S., Charrier J.-P., Choquet-Kastylevsky G., Rolland D., et al. (2013). Protein disulfide isomerase assay method for the in vitro diagnosis of colorectal cancer. Patents 1–30.
-
- Atkin J. D., Farg M. A., Turner B. J., Tomas D., Lysaght J. A., Cheema S. S., et al. . (2006). Induction of the unfolded protein response in familial amyotrophic lateral sclerosis and association of protein-disulfide isomerase with superoxide dismutase 1. J. Biol. Chem. 281, 30152–30165. 10.1074/jbc.M603393200 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

