Re-examination of regulatory opinions in Europe: possible contribution for the approval of the first gene therapy product Glybera
- PMID: 26052534
- PMCID: PMC4449026
- DOI: 10.1038/mtm.2014.66
Re-examination of regulatory opinions in Europe: possible contribution for the approval of the first gene therapy product Glybera
Abstract
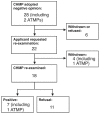
The first commercially approved human gene therapy in the Western world is Glybera (alipogene tiparvovec), which is an adenoassociated viral vector encoding the lipoprotein lipase gene. Glybera was recommended for marketing authorization by the European Medicines Agency in 2012. The European Medicines Agency had only ever reviewed three marketing authorization applications for gene therapy medicinal products. Unlike in the case of Glybera, the applications of the first two products, Cerepro and Contusugene Ladenovec Gendux/Advexin, both of which were for cancer diseases, were withdrawn. In this report, we studied the European public assessment reports of the three gene therapy products. During the assessment process, Glybera was re-examined and reviewed for a fourth time. We therefore researched the re-examination procedure of the European Union regulatory process. Approximately 25% of the new medicinal products initially given negative opinions from the Committee for Medicinal Products for Human Use were ultimately approved after re-examination from 2009 to 2013. The indications of most medicines were changed during the re-examination procedure, and the products were later approved with a mode of approval. These results suggested that the re-examination system in the European Union contributed to the approval of both several new drugs and the first gene therapy product.
Figures

References
-
- Wirth T, Parker N, Ylä-Herttuala S. History of gene therapy. Gene. 2013;525:162–169. - PubMed
-
- Gaudet D, Méthot J, Kastelein J. Gene therapy for lipoprotein lipase deficiency. Curr Opin Lipidol. 2012;23:310–320. - PubMed
-
- Official Journal of the European Union Regulation (EC) No 1394/2007 of The European Parliament and of the Council of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004 . < http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:324:0121... > ( 2007
LinkOut - more resources
Full Text Sources
Other Literature Sources

