Where hearing starts: the development of the mammalian cochlea
- PMID: 26052920
- PMCID: PMC4718162
- DOI: 10.1111/joa.12314
Where hearing starts: the development of the mammalian cochlea
Abstract
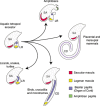
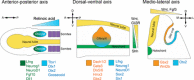
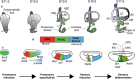
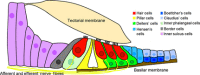
The mammalian cochlea is a remarkable sensory organ, capable of perceiving sound over a range of 10(12) in pressure, and discriminating both infrasonic and ultrasonic frequencies in different species. The sensory hair cells of the mammalian cochlea are exquisitely sensitive, responding to atomic-level deflections at speeds on the order of tens of microseconds. The number and placement of hair cells are precisely determined during inner ear development, and a large number of developmental processes sculpt the shape, size and morphology of these cells along the length of the cochlear duct to make them optimally responsive to different sound frequencies. In this review, we briefly discuss the evolutionary origins of the mammalian cochlea, and then describe the successive developmental processes that lead to its induction, cell cycle exit, cellular patterning and the establishment of topologically distinct frequency responses along its length.
Keywords: BMP; Cochlea; FGF; Hair cells; Notch; Organ of Corti; Sensory; Shh; tonotopy.
© 2015 Anatomical Society.
Figures

References
-
- Abdelalim EM, Emara MM, Kolatkar PR (2014) The SOX transcription factors as key players in pluripotent stem cells. Stem Cells Dev 23, 2687–2699. - PubMed
-
- Adam J, Myat A, Le Roux I, et al. (1998) Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense‐organ development. Development 125, 4645–4654. - PubMed
-
- Affolter M, Basler K (2007) The Decapentaplegic morphogen gradient: from pattern formation to growth regulation. Nat Rev Genet 8, 663–674. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

