Hippocampal-prefrontal input supports spatial encoding in working memory
- PMID: 26053122
- PMCID: PMC4505751
- DOI: 10.1038/nature14445
Hippocampal-prefrontal input supports spatial encoding in working memory
Abstract
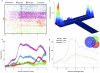
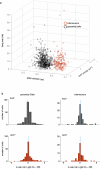
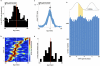
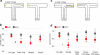
Spatial working memory, the caching of behaviourally relevant spatial cues on a timescale of seconds, is a fundamental constituent of cognition. Although the prefrontal cortex and hippocampus are known to contribute jointly to successful spatial working memory, the anatomical pathway and temporal window for the interaction of these structures critical to spatial working memory has not yet been established. Here we find that direct hippocampal-prefrontal afferents are critical for encoding, but not for maintenance or retrieval, of spatial cues in mice. These cues are represented by the activity of individual prefrontal units in a manner that is dependent on hippocampal input only during the cue-encoding phase of a spatial working memory task. Successful encoding of these cues appears to be mediated by gamma-frequency synchrony between the two structures. These findings indicate a critical role for the direct hippocampal-prefrontal afferent pathway in the continuous updating of task-related spatial information during spatial working memory.
Figures








References
-
- Baddeley A, Hitch G. In: Recent advances in learning and motivation. Bower GA, editor. Vol. 8. Academic Press; 1974. pp. 47–90.
-
- Klauer K, Zhao Z. Double dissociations in visual and spatial short-term memory. Journal of Experimental Psychology: General. 2004;133:355–381. - PubMed
-
- Andrade J. Working Memory in Context. Psychology; 2001.
-
- Miyake A, Shah P. Models of Working Memory: Mechanisms of Active Maintenance and Executive Control. Cambridge University Press; 1999.
-
- Baddeley A. Working memory: looking back and looking forward. Nature Reviews Neuroscience. 2003;4:829–839. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

