Tracks through the genome to physiological events
- PMID: 26053180
- PMCID: PMC5008151
- DOI: 10.1113/EP085129
Tracks through the genome to physiological events
Abstract
What is the topic of this review? We discuss tools available to access genome-wide data sets that harbour cell-specific, brain region-specific and tissue-specific information on exon usage for several species, including humans. In this Review, we demonstrate how to access this information in genome databases and its enormous value to physiology. What advances does it highlight? The sheer scale of protein diversity that is possible from complex genes, including those that encode voltage-gated ion channels, is vast. But this choice is critical for a complete understanding of protein function in the most physiologically relevant context. Many proteins of great interest to physiologists and neuroscientists are structurally complex and located in specialized subcellular domains, such as neuronal synapses and transverse tubules of muscle. Genes that encode these critical signalling molecules (receptors, ion channels, transporters, enzymes, cell adhesion molecules, cell-cell interaction proteins and cytoskeletal proteins) are similarly complex. Typically, these genes are large; human Dystrophin (DMD) encodes a cytoskeletal protein of muscle and it is the largest naturally occurring gene at a staggering 2.3 Mb. Large genes contain many non-coding introns and coding exons; human Titin (TTN), which encodes a protein essential for the assembly and functioning of vertebrate striated muscles, has over 350 exons and consequently has an enormous capacity to generate different forms of Titin mRNAs that have unique exon combinations. Functional and pharmacological differences among protein isoforms originating from the same gene may be subtle but nonetheless of critical physiological significance. Standard functional, immunological and pharmacological approaches, so useful for characterizing proteins encoded by different genes, typically fail to discriminate among splice isoforms of individual genes. Tools are now available to access genome-wide data sets that harbour cell-specific, brain region-specific and tissue-specific information on exon usage for several species, including humans. In this Review, we demonstrate how to access this information in genome databases and its enormous value to physiology.
© 2015 The Authors. Experimental Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures
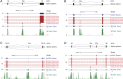
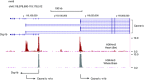
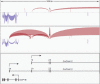

References
-
- Achsel T, Barabino S, Cozzolino M & Carri MT (2013). The intriguing case of motor neuron disease: ALS and SMA come closer. Biochem Soc Trans 41, 1593–1597. - PubMed
-
- Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, Thurman RE, Kuehn MS, Taylor CM, Neph S, Koch CM, Asthana S, Malhotra A, Adzhubei I, Greenbaum JA, Andrews RM, Flicek P, Boyle PJ, Cao H, Carter NP, Clelland GK, Davis S, Day N, Dhami P, Dillon SC, Dorschner MO, Fiegler H, Giresi PG, Goldy J, Hawrylycz M, Haydock A, Humbert R, James KD, Johnson BE, Johnson EM, Frum TT, Rosenzweig ER, Karnani N, Lee K, Lefebvre GC, Navas PA, Neri F, Parker SC, Sabo PJ, Sandstrom R, Shafer A, Vetrie D, Weaver M, Wilcox S, Yu M, Collins FS, Dekker J, Lieb JD, Tullius TD, Crawford GE, Sunyaev S, Noble WS, Dunham I, Denoeud F, Reymond A, Kapranov P, Rozowsky J, Zheng D, Castelo R, Frankish A, Harrow J, Ghosh S, Sandelin A, Hofacker IL, Baertsch R, Keefe D, Dike S, Cheng J, Hirsch HA, Sekinger EA, Lagarde J, Abril JF, Shahab A, Flamm C, Fried C, Hackermuller J, Hertel J, Lindemeyer M, Missal K, Tanzer A, Washietl S, Korbel J, Emanuelsson O, Pedersen JS, Holroyd N, Taylor R, Swarbreck D, Matthews N, Dickson MC, Thomas DJ, Weirauch MT, Gilbert J, Drenkow J, Bell I, Zhao X, Srinivasan KG, Sung WK, Ooi HS, Chiu KP, Foissac S, Alioto T, Brent M, Pachter L, Tress ML, Valencia A, Choo SW, Choo CY, Ucla C, Manzano C, Wyss C, Cheung E, Clark TG, Brown JB, Ganesh M, Patel S, Tammana H, Chrast J, Henrichsen CN, Kai C, Kawai J, Nagalakshmi U, Wu J, Lian Z, Lian J, Newburger P, Zhang X, Bickel P, Mattick JS, Carninci P, Hayashizaki Y, Weissman S, Hubbard T, Myers RM, Rogers J, Stadler PF, Lowe TM, Wei CL, Ruan Y, Struhl K, Gerstein M, Antonarakis SE, Fu Y, Green ED, Karaoz U, Siepel A, Taylor J, Liefer LA, Wetterstrand KA, Good PJ, Feingold EA, Guyer MS, Cooper GM, Asimenos G, Dewey CN, Hou M, Nikolaev S, Montoya‐Burgos JI, Loytynoja A, Whelan S, Pardi F, Massingham T, Huang H, Zhang NR, Holmes I, Mullikin JC, Ureta‐Vidal A, Paten B, Seringhaus M, Church D, Rosenbloom K, Kent WJ, Stone EA, Batzoglou S, Goldman N, Hardison RC, Haussler D, Miller W, Sidow A, Trinklein ND, Zhang ZD, Barrera L, Stuart R, King DC, Ameur A, Enroth S, Bieda MC, Kim J, Bhinge AA, Jiang N, Liu J, Yao F, Vega VB, Lee CW, Ng P, Shahab A, Yang A, Moqtaderi Z, Zhu Z, Xu X, Squazzo S, Oberley MJ, Inman D, Singer MA, Richmond TA, Munn KJ, Rada‐Iglesias A, Wallerman O, Komorowski J, Fowler JC, Couttet P, Bruce AW, Dovey OM, Ellis PD, Langford CF, Nix DA, Euskirchen G, Hartman S, Urban AE, Kraus P, Van Calcar S, Heintzman N, Kim TH, Wang K, Qu C, Hon G, Luna R, Glass CK, Rosenfeld MG, Aldred SF, Cooper SJ, Halees A, Lin JM, Shulha HP, Zhang X, Xu M, Haidar JN, Yu Y, Ruan Y, Iyer VR, Green RD, Wadelius C, Farnham PJ, Ren B, Harte RA, Hinrichs AS, Trumbower H, Clawson H, Hillman‐Jackson J, Zweig AS, Smith K, Thakkapallayil A, Barber G, Kuhn RM, Karolchik D, Armengol L, Bird CP, de Bakker PI, Kern AD, Lopez‐Bigas N, Martin JD, Stranger BE, Woodroffe A, Davydov E, Dimas A, Eyras E, Hallgrimsdottir IB, Huppert J, Zody MC, Abecasis GR, Estivill X, Bouffard GG, Guan X, Hansen NF, Idol JR, Maduro VV, Maskeri B, McDowell JC, Park M, Thomas PJ, Young AC, Blakesley RW, Muzny DM, Sodergren E, Wheeler DA, Worley KC, Jiang H, Weinstock GM, Gibbs RA, Graves T, Fulton R, Mardis ER, Wilson RK, Clamp M, Cuff J, Gnerre S, Jaffe DB, Chang JL, Lindblad‐Toh K, Lander ES, Koriabine M, Nefedov M, Osoegawa K, Yoshinaga Y, Zhu B & de Jong PJ (2007). Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447, 799–816. - PMC - PubMed
-
- BrainSpan (2011). Atlas of the developing human brain. Funded by ARRA Awards 1RC2MH089921‐01, 1RC2MH090047‐01 and 1RC2MH089929‐01. Available from: http://developinghumanbrain.org
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

