Prebiotics Modulate the Effects of Antibiotics on Gut Microbial Diversity and Functioning in Vitro
- PMID: 26053617
- PMCID: PMC4488797
- DOI: 10.3390/nu7064480
Prebiotics Modulate the Effects of Antibiotics on Gut Microbial Diversity and Functioning in Vitro
Abstract
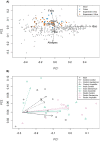
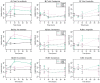
Intestinal bacteria carry out many fundamental roles, such as the fermentation of non-digestible dietary carbohydrates to produce short chain fatty acids (SCFAs), which can affect host energy levels and gut hormone regulation. Understanding how to manage this ecosystem to improve human health is an important but challenging goal. Antibiotics are the front line of defence against pathogens, but in turn they have adverse effects on indigenous microbial diversity and function. Here, we have investigated whether dietary supplementation--another method used to modulate gut composition and function--could be used to ameliorate the side effects of antibiotics. We perturbed gut bacterial communities with gentamicin and ampicillin in anaerobic batch cultures in vitro. Cultures were supplemented with either pectin (a non-fermentable fibre), inulin (a commonly used prebiotic that promotes the growth of beneficial bacteria) or neither. Although antibiotics often negated the beneficial effects of dietary supplementation, in some treatment combinations, notably ampicillin and inulin, dietary supplementation ameliorated the effects of antibiotics. There is therefore potential for using supplements to lessen the adverse effects of antibiotics. Further knowledge of such mechanisms could lead to better therapeutic manipulation of the human gut microbiota.
Keywords: antibiotics; fibre; gut microbiota; prebiotics.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

