Harnessing endogenous stem/progenitor cells for tendon regeneration
- PMID: 26053662
- PMCID: PMC4563693
- DOI: 10.1172/JCI81589
Harnessing endogenous stem/progenitor cells for tendon regeneration
Abstract
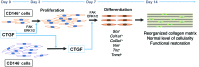
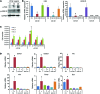
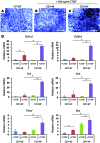
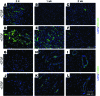
Current stem cell-based strategies for tissue regeneration involve ex vivo manipulation of these cells to confer features of the desired progenitor population. Recently, the concept that endogenous stem/progenitor cells could be used for regenerating tissues has emerged as a promising approach that potentially overcomes the obstacles related to cell transplantation. Here we applied this strategy for the regeneration of injured tendons in a rat model. First, we identified a rare fraction of tendon cells that was positive for the known tendon stem cell marker CD146 and exhibited clonogenic capacity, as well as multilineage differentiation ability. These tendon-resident CD146+ stem/progenitor cells were selectively enriched by connective tissue growth factor delivery (CTGF delivery) in the early phase of tendon healing, followed by tenogenic differentiation in the later phase. The time-controlled proliferation and differentiation of CD146+ stem/progenitor cells by CTGF delivery successfully led to tendon regeneration with densely aligned collagen fibers, normal level of cellularity, and functional restoration. Using siRNA knockdown to evaluate factors involved in tendon generation, we demonstrated that the FAK/ERK1/2 signaling pathway regulates CTGF-induced proliferation and differentiation of CD146+ stem/progenitor cells. Together, our findings support the use of endogenous stem/progenitor cells as a strategy for tendon regeneration without cell transplantation and suggest this approach warrants exploration in other tissues.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

