Alternatives to Hazard Ratios for Comparing the Efficacy or Safety of Therapies in Noninferiority Studies
- PMID: 26054047
- PMCID: PMC4510023
- DOI: 10.7326/M14-1741
Alternatives to Hazard Ratios for Comparing the Efficacy or Safety of Therapies in Noninferiority Studies
Abstract
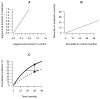
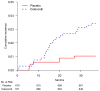
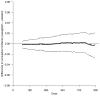
A noninferiority study is often used to investigate whether a treatment's efficacy or safety profile is acceptable compared with an alternative therapy regarding the time to a clinical event. The empirical quantification of the treatment difference for such a study is routinely based on the hazard ratio (HR) estimate. The HR, which is not a relative risk, may be difficult to interpret clinically, especially when the underlying proportional hazards assumption is violated. The precision of the HR estimate depends primarily on the number of observed events but not directly on exposure times or sample size of the study population. If the event rate is low, the study may require an impractically large number of events to ensure that the prespecified noninferiority criterion for the HR is attainable. This article discusses deficiencies in the current approach for the design and analysis of a noninferiority study. Alternative procedures are provided, which do not depend on any model assumption, to compare 2 treatments. For a noninferiority safety study, the patients' exposure times are more clinically important than the observed number of events. If the patients' exposure times are long enough to evaluate safety reliably, then these alternative procedures can effectively provide clinically interpretable evidence on safety, even with relatively few observed events. These procedures are illustrated with data from 2 studies. One explores the cardiovascular safety of a pain medicine; the second examines the cardiovascular safety of a new treatment for diabetes. These alternative strategies to evaluate safety or efficacy of an intervention lead to more meaningful interpretations of the analysis results than the conventional strategy that uses the HR estimate.
Figures

References
-
- D’Agostino RB, Massaro JM, Sullivan LM. Non-inferiority trials: design concepts and issues - the encounters of academic consultants in statistics. Stat Med. 2003;22:169–86. - PubMed
-
- Head SJ, Kaul S, Bogers AJ, Kappetein AP. Non-inferiority study design: lessons to be learned from cardiovascular trials. Eur Heart J. 2012;33:1318–24. - PubMed
References for Appendices
-
- Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus. N Engl J Med. 2013;369:1317–26. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
