The developmental evolution of the seizure phenotype and cortical inhibition in mouse models of juvenile myoclonic epilepsy
- PMID: 26054439
- PMCID: PMC4641014
- DOI: 10.1016/j.nbd.2015.05.016
The developmental evolution of the seizure phenotype and cortical inhibition in mouse models of juvenile myoclonic epilepsy
Abstract
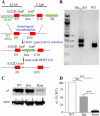
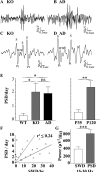
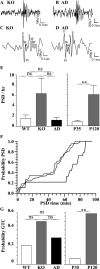
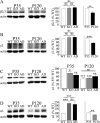
The GABA(A) receptor (GABA(A)R) α1 subunit mutation, A322D, causes autosomal dominant juvenile myoclonic epilepsy (JME). Previous in vitro studies demonstrated that A322D elicits α1(A322D) protein degradation and that the residual mutant protein causes a dominant-negative effect on wild type GABA(A)Rs. Here, we determined the effects of heterozygous A322D knockin (Het(α1)AD) and deletion (Het(α1)KO) on seizures, GABA(A)R expression, and motor cortex (M1) miniature inhibitory postsynaptic currents (mIPSCs) at two developmental time-points, P35 and P120. Both Het(α1)AD and Het(α1)KO mice experience absence seizures at P35 that persist at P120, but have substantially more frequent spontaneous and evoked polyspike wave discharges and myoclonic seizures at P120. Both mutant mice have increased total and synaptic α3 subunit expression at both time-points and decreased α1 subunit expression at P35, but not P120. There are proportional reductions in α3, β2, and γ2 subunit expression between P35 and P120 in wild type and mutant mice. In M1, mutants have decreased mIPSC peak amplitudes and prolonged decay constants compared with wild type, and the Het(α1)AD mice have reduced mIPSC frequency and smaller amplitudes than Het(α1)KO mice. Wild type and mutants exhibit proportional increases in mIPSC amplitudes between P35 and P120. We conclude that Het(α1)KO and Het(α1)AD mice model the JME subsyndrome, childhood absence epilepsy persisting and evolving into JME. Both mutants alter GABA(A)R composition and motor cortex physiology in a manner expected to increase neuronal synchrony and excitability to produce seizures. However, developmental changes in M1 GABA(A)Rs do not explain the worsened phenotype at P120 in mutant mice.
Keywords: Brain; Confocal microscopy; Electroencephalography; Electrophysiology; Immunofluorescence; Patch-clamp; Western blot.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures



References
-
- Akman O, Demiralp T, Ates N, Onat FY. Electroencephalographic differences between WAG/Rij and GAERS rat models of absence epilepsy. Epilepsy Res. 2010;89:185–193. - PubMed
-
- Badawy RA, Vogrin SJ, Lai A, Cook MJ. Patterns of cortical hyperexcitability in adolescent/adult-onset generalized epilepsies. Epilepsia. 2013;54:871–878. - PubMed
-
- Bartolini E, Pesaresi I, Fabbri S, Cecchi P, Giorgi FS, Sartucci F, et al. Abnormal response to photic stimulation in Juvenile Myoclonic Epilepsy: An EEG-fMRI study. Epilepsia. 2014 - PubMed
-
- Benarroch EE. GABAA receptor heterogeneity, function, and implications for epilepsy. Neurology. 2007;68:612–614. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

