Surveillance of γδ T Cells Predicts Cytomegalovirus Infection Resolution in Kidney Transplants
- PMID: 26054538
- PMCID: PMC4731109
- DOI: 10.1681/ASN.2014100985
Surveillance of γδ T Cells Predicts Cytomegalovirus Infection Resolution in Kidney Transplants
Abstract
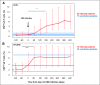
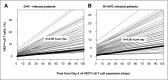
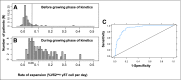
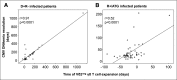
Cytomegalovirus (CMV) infection in solid-organ transplantation is associated with increased morbidity and mortality, particularly if a CMV mutant strain with antiviral resistance emerges. Monitoring CMV-specific T cell response could provide relevant information for patient care. We and others have shown the involvement of Vδ2(neg) γδ T cells in controlling CMV infection. Here, we assessed if Vδ2(neg) γδ T cell kinetics in peripheral blood predict CMV infection resolution and emergence of a mutant strain in high-risk recipients of kidney transplants, including 168 seronegative recipients receiving organs from seropositive donors (D+R-) and 104 seropositive recipients receiving antithymocyte globulins (R+/ATG). Vδ2(neg) γδ T cell percentages were serially determined in patients grafted between 2003 and 2011. The growing phase of Vδ2(neg) γδ T cells was monitored in each infected patient, and the expansion rate during this phase was estimated individually by a linear mixed model. A Vδ2(neg) γδ T cell expansion rate of ˃0.06% per day predicted the growing phase. The time after infection at which an expansion rate of 0.06% per day occurred was correlated with the resolution of CMV DNAemia (r=0.91; P<0.001). At 49 days of antiviral treatment, Vδ2(neg) γδ T cell expansion onset was associated with recovery, whereas absence of expansion was associated with recurrent disease and DNAemia. The appearance of antiviral-resistant mutant CMV strains was associated with delayed Vδ2(neg) γδ T cell expansion (P<0.001). In conclusion, longitudinal surveillance of Vδ2(neg) γδ T cells in recipients of kidney transplants may predict CMV infection resolution and antiviral drug resistance.
Keywords: cytomegalovirus; immunology; immunosuppression; kidney transplantation.
Copyright © 2016 by the American Society of Nephrology.
Figures



References
-
- Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, Humar A, Transplantation Society International CMV Consensus Group : Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 96: 333–360, 2013 - PubMed
-
- Asberg A, Humar A, Rollag H, Jardine AG, Mouas H, Pescovitz MD, Sgarabotto D, Tuncer M, Noronha IL, Hartmann A, VICTOR Study Group : Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant 7: 2106–2113, 2007 - PubMed
-
- Asberg A, Humar A, Jardine AG, Rollag H, Pescovitz MD, Mouas H, Bignamini A, Töz H, Dittmer I, Montejo M, Hartmann A, VICTOR Study Group : Long-term outcomes of CMV disease treatment with valganciclovir versus IV ganciclovir in solid organ transplant recipients. Am J Transplant 9: 1205–1213, 2009 - PubMed
-
- Le Page AK, Jager MM, Iwasenko JM, Scott GM, Alain S, Rawlinson WD: Clinical aspects of cytomegalovirus antiviral resistance in solid organ transplant recipients. Clin Infect Dis 56: 1018–1029, 2013 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

