CKD Stimulates Muscle Protein Loss Via Rho-associated Protein Kinase 1 Activation
- PMID: 26054539
- PMCID: PMC4731120
- DOI: 10.1681/ASN.2014121208
CKD Stimulates Muscle Protein Loss Via Rho-associated Protein Kinase 1 Activation
Abstract
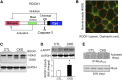
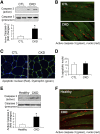
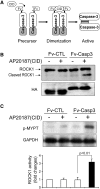
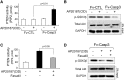
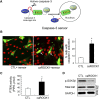
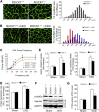
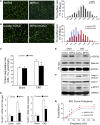
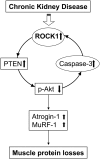
In patients with CKD, muscle wasting is common and is associated with morbidity and mortality. Mechanisms leading to loss of muscle proteins include insulin resistance, which suppresses Akt activity and thus stimulates protein degradation via the ubiquitin-proteasome system. However, the specific factors controlling CKD-induced suppression of Akt activity in muscle remain undefined. In mice with CKD, the reduction in Akt activity in muscle exceeded the decrease in upstream insulin receptor substrate-1-associated phosphatidylinositol 3-kinase activity, suggesting that CKD activates other pathways that suppress Akt. Furthermore, a CKD-induced increase uncovered caspase-3 activity in muscle in these mice. In C2C12 muscle cells, activated caspase-3 cleaves and activates Rho-associated protein kinase 1 (ROCK1), which enhances the activity of phosphatase and tensin homolog (PTEN) and reduces Akt activity. Notably, constitutive activation of ROCK1 also led to increased caspase-3 activity in vitro. In mice with either global ROCK1 knockout or muscle-specific PTEN knockout, CKD-associated muscle proteolysis was blunted. These results suggest ROCK1 activation in CKD and perhaps in other catabolic conditions can promote loss of muscle protein via a negative feedback loop.
Keywords: cell signaling; chronic kidney disease; metabolism.
Copyright © 2016 by the American Society of Nephrology.
Figures

References
-
- Kopple JD, Wang H, Casaburi R, Fournier M, Lewis MI, Taylor W, Storer TW: Exercise in maintenance hemodialysis patients induces transcriptional changes in genes favoring anabolic muscle. J Am Soc Nephrol 18: 2975–2986, 2007 - PubMed
-
- Cheung WW, Paik KH, Mak RH: Inflammation and cachexia in chronic kidney disease. Pediatr Nephrol 25: 711–724, 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

