Rapid method for the isolation of mammalian sperm DNA
- PMID: 26054765
- PMCID: PMC4486329
- DOI: 10.2144/000114280
Rapid method for the isolation of mammalian sperm DNA
Abstract
The unique DNA packaging of spermatozoa renders them resistant to DNA isolation techniques used for somatic cells, requiring alternative methods that are slow and labor intensive. Here we present a rapid method for isolating high-quality sperm DNA. Isolated human sperm cells were homogenized with 0.2 mm steel beads for 5 min at room temperature in the presence of guanidine thiocyanate lysis buffer supplemented with 50 mM tris(2-carboxyethyl)phosphine (TCEP). Our method yielded >90% high-quality DNA using 3 different commercially available silica-based spin columns. DNA yields did not differ between immediate isolation (2.84 ± 0.04 pg/cell) and isolation after 2 weeks of homogenate storage at room temperature (2.91 ± 0.13 pg/cell). DNA methylation analyses revealed similar methylation levels at both time points for three imprinted loci. Our protocol has many advantages: it is conducted at room temperature; lengthy proteinase K (ProK) digestions are eliminated; the reducing agent, TCEP, is odorless and stable at room temperature; nucleic acids are stabilized, allowing storage of homogenate; and it is adaptable for other mammalian species. Taken together, the benefits of our improved method have important implications for settings where sample processing constraints exist.
Keywords: DNA isolation; DNA purification; epigenetics; genetics; methods; nucleic acids; sperm; spermatozoa.
Figures
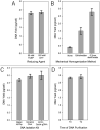
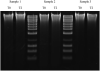
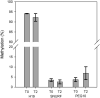
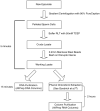
References
-
- Kobayashi H, Sato A, Otsu E, Hiura H, Tomatsu C, Utsunomiya T, Sasaki H, Yaegashi N, Arima T. Aberrant DNA methylation of imprinted loci in sperm from oligospermic patients. Hum. Mol. Genet. 2007;16:2542–2551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
