Energizing eukaryotic cell-free protein synthesis with glucose metabolism
- PMID: 26054976
- PMCID: PMC4651010
- DOI: 10.1016/j.febslet.2015.05.045
Energizing eukaryotic cell-free protein synthesis with glucose metabolism
Abstract
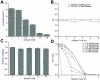
Eukaryotic cell-free protein synthesis (CFPS) is limited by the dependence on costly high-energy phosphate compounds and exogenous enzymes to power protein synthesis (e.g., creatine phosphate and creatine kinase, CrP/CrK). Here, we report the ability to use glucose as a secondary energy substrate to regenerate ATP in a Saccharomyces cerevisiae crude extract CFPS platform. We observed synthesis of 3.64±0.35 μg mL(-1) active luciferase in batch reactions with 16 mM glucose and 25 mM phosphate, resulting in a 16% increase in relative protein yield (μg protein/$ reagents) compared to the CrP/CrK system. Our demonstration provides the foundation for development of cost-effective eukaryotic CFPS platforms.
Keywords: Cell-free biology; Cell-free protein synthesis; In vitro transcription and translation; Natural energy metabolism; Protein expression; Saccharomyces cerevisiae.
Copyright © 2015 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures

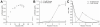

References
-
- Atkinson DE. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968;7:4030–4034. - PubMed
-
- Buchner E, Rapp R. Alkoholische gährung ohne hefezellen. Berichte der deutschen chemischen Gesellschaft. 1897;30:2668–2678.
-
- Bujara M, Schumperli M, Billerbeck S, Heinemann M, Panke S. Exploiting cell-free systems: Implementation and debugging of a system of biotransformations. Biotechnol Bioeng. 2010;106:376–389. - PubMed
-
- Calhoun KA, Swartz JR. An economical method for cell-free protein synthesis using glucose and nucleoside monophosphates. Biotechnology progress. 2005a;21:1146–1153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

