Infection of ectocervical tissue and universal targeting of T-cells mediated by primary non-macrophage-tropic and highly macrophage-tropic HIV-1 R5 envelopes
- PMID: 26055104
- PMCID: PMC4459458
- DOI: 10.1186/s12977-015-0176-2
Infection of ectocervical tissue and universal targeting of T-cells mediated by primary non-macrophage-tropic and highly macrophage-tropic HIV-1 R5 envelopes
Abstract
Background: HIV-1 variants carrying non-macrophage-tropic HIV-1 R5 envelopes (Envs) are predominantly transmitted and persist in immune tissue even in AIDS patients who have highly macrophage-tropic variants in the brain. Non-macrophage-tropic R5 Envs require high levels of CD4 for infection contrasting with macrophage-tropic Envs, which can efficiently mediate infection of cells via low CD4. Here, we investigated whether non-macrophage-tropic R5 Envs from the acute stage of infection (including transmitted/founder Env) mediated more efficient infection of ectocervical explant cultures compared to non-macrophage-tropic and highly macrophage-tropic R5 Envs from late disease.
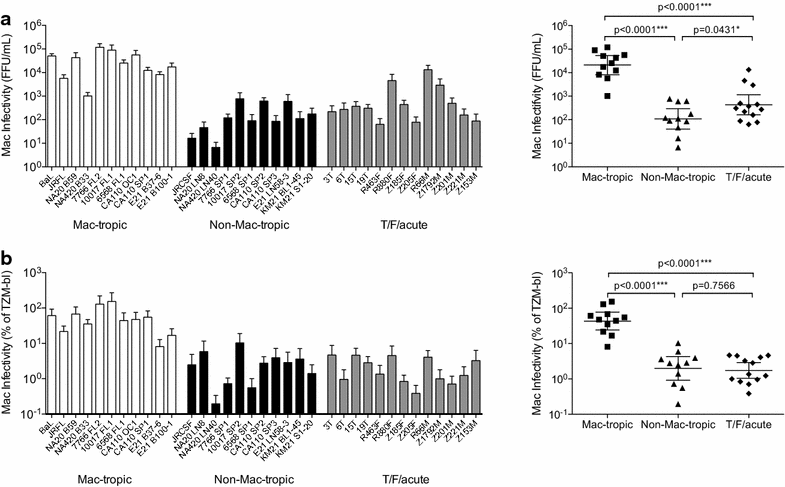
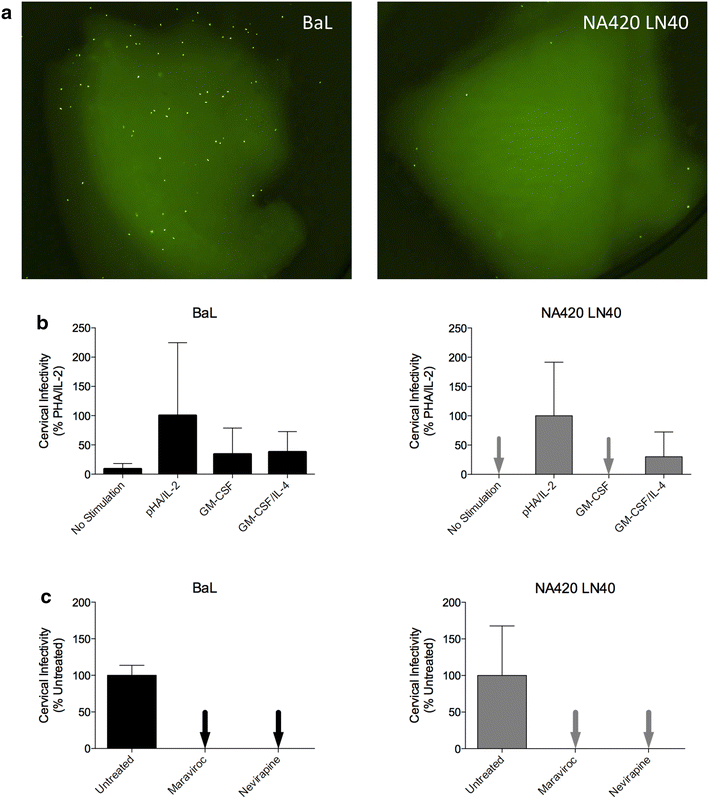
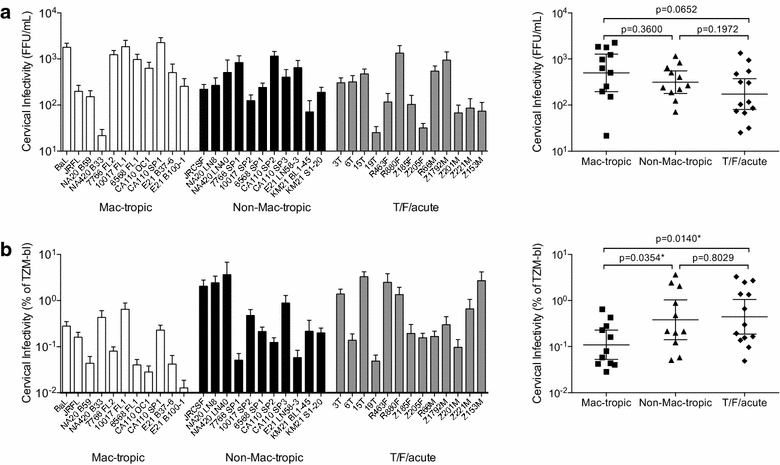
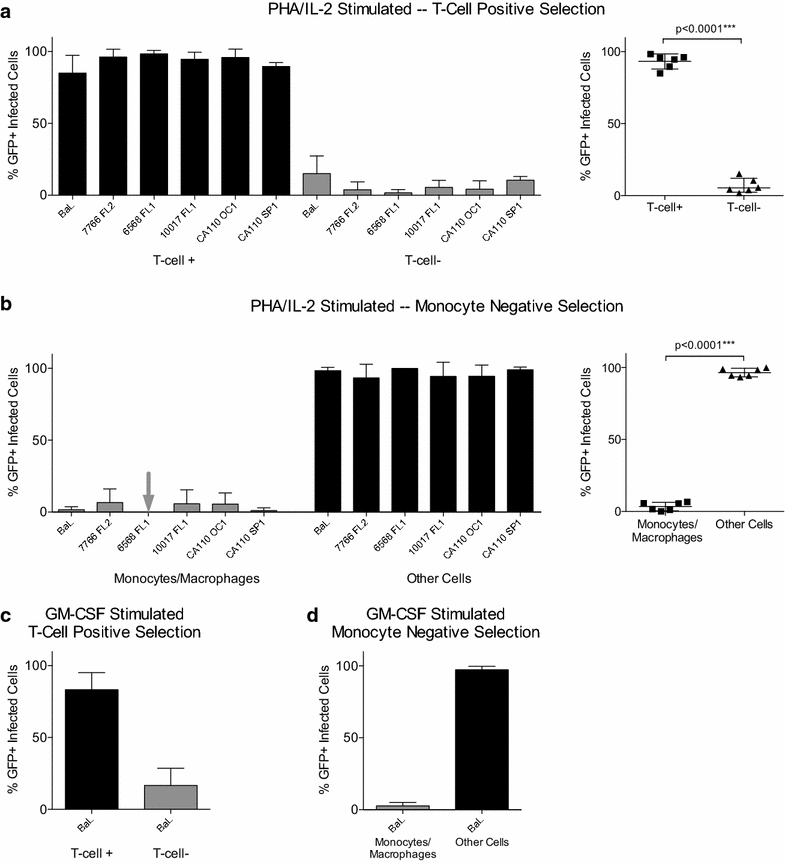
Results: We used Env+ pseudovirions that carried a GFP reporter gene to measure infection of the first cells targeted in ectocervical explant cultures. In straight titrations of Env+ pseudovirus supernatants, mac-tropic R5 Envs from late disease mediated slightly higher infectivities for ectocervical explants although this was not significant. Surprisingly, explant infection by several T/F/acute Envs was lower than for Envs from late disease. However, when infectivity for explants was corrected to account for differences in the overall infectivity of each Env+ pseudovirus (measured on highly permissive HeLa TZM-bl cells), non-mac-tropic early and late disease Env+ pseudoviruses mediated significantly higher infection. This observation suggests that cervical tissue preferentially supports non-mac-tropic Env+ viruses compared to mac-tropic viruses. Finally, we show that T-cells were the main targets for infection regardless of whether explants were stimulated with T-cell or monocyte/macrophage cytokines. There was no evidence of macrophage infection even for pseudovirions carrying highly mac-tropic Envs from brain tissue or for the highly mac-tropic, laboratory strain, BaL, which targeted T-cells in the explant tissue.
Conclusions: Our data support ectocervical tissue as a favorable environment for non-mac-tropic HIV-1 R5 variants and emphasize the role of T-cells as initial targets for infection even for highly mac-tropic variants.
Figures
References
-
- Abrahams MR, Anderson JA, Giorgi EE, Seoighe C, Mlisana K, Ping LH, et al. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-poisson distribution of transmitted variants. J Virol. 2009;83:3556–3567. doi: 10.1128/JVI.02132-08. - DOI - PMC - PubMed
-
- Peters PJ, Bhattacharya J, Hibbitts S, Dittmar MT, Simmons G, Bell J, et al. Biological analysis of human immunodeficiency virus type 1 R5 envelopes amplified from brain and lymph node tissues of AIDS patients with neuropathology reveals two distinct tropism phenotypes and identifies envelopes in the brain that confer an enhanced tropism and fusigenicity for macrophages. J Virol. 2004;78:6915–6926. doi: 10.1128/JVI.78.13.6915-6926.2004. - DOI - PMC - PubMed
-
- Peters PJ, Sullivan WM, Duenas-Decamp MJ, Bhattacharya J, Ankghuambom C, Brown R, et al. Non-macrophage-tropic human immunodeficiency virus type 1 R5 envelopes predominate in blood, lymph nodes, and semen: implications for transmission and pathogenesis. J Virol. 2006;80:6324–6332. doi: 10.1128/JVI.02328-05. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

