TRIM28 Is an E3 Ligase for ARF-Mediated NPM1/B23 SUMOylation That Represses Centrosome Amplification
- PMID: 26055329
- PMCID: PMC4508312
- DOI: 10.1128/MCB.01064-14
TRIM28 Is an E3 Ligase for ARF-Mediated NPM1/B23 SUMOylation That Represses Centrosome Amplification
Abstract
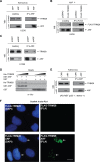
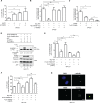
The tumor suppressor ARF enhances the SUMOylation of target proteins; however, the physiological function of ARF-mediated SUMOylation has been unclear due to the lack of a known, associated E3 SUMO ligase. Here we uncover TRIM28/KAP1 as a novel ARF-binding protein and SUMO E3 ligase for NPM1/B23. ARF and TRIM28 cooperate to SUMOylate NPM1, a nucleolar protein that regulates centrosome duplication and genomic stability. ARF-mediated SUMOylation of NPM1 was attenuated by TRIM28 depletion and enhanced by TRIM28 overexpression. Coexpression of ARF and TRIM28 promoted NPM1 centrosomal localization by enhancing its SUMOylation and suppressed centrosome amplification; these functions required the E3 ligase activity of TRIM28. Conversely, depletion of ARF or TRIM28 increased centrosome amplification. ARF also counteracted oncogenic Ras-induced centrosome amplification. Centrosome amplification is often induced by oncogenic insults, leading to genomic instability. However, the mechanisms employed by tumor suppressors to protect the genome are poorly understood. Our findings suggest a novel role for ARF in maintaining genome integrity by facilitating TRIM28-mediated SUMOylation of NPM1, thus preventing centrosome amplification.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
