Validation of an automated seizure detection algorithm for term neonates
- PMID: 26055336
- PMCID: PMC4727504
- DOI: 10.1016/j.clinph.2015.04.075
Validation of an automated seizure detection algorithm for term neonates
Abstract
Objective: The objective of this study was to validate the performance of a seizure detection algorithm (SDA) developed by our group, on previously unseen, prolonged, unedited EEG recordings from 70 babies from 2 centres.
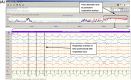
Methods: EEGs of 70 babies (35 seizure, 35 non-seizure) were annotated for seizures by experts as the gold standard. The SDA was tested on the EEGs at a range of sensitivity settings. Annotations from the expert and SDA were compared using event and epoch based metrics. The effect of seizure duration on SDA performance was also analysed.
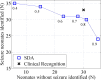
Results: Between sensitivity settings of 0.5 and 0.3, the algorithm achieved seizure detection rates of 52.6-75.0%, with false detection (FD) rates of 0.04-0.36FD/h for event based analysis, which was deemed to be acceptable in a clinical environment. Time based comparison of expert and SDA annotations using Cohen's Kappa Index revealed a best performing SDA threshold of 0.4 (Kappa 0.630). The SDA showed improved detection performance with longer seizures.
Conclusion: The SDA achieved promising performance and warrants further testing in a live clinical evaluation.
Significance: The SDA has the potential to improve seizure detection and provide a robust tool for comparing treatment regimens.
Keywords: Automated seizure detection; Hypoxic-ischaemic encephalopathy; Neonatal EEG; Neonatal neurology; Neonatal seizures.
Copyright © 2015 International Federation of Clinical Neurophysiology. Published by Elsevier Ireland Ltd. All rights reserved.
Figures













References
-
- Cherian P.J., Deburchgraeve W., Swarte R.M., De Vos M., Govaert P., Van Huffel S. Validation of a new automated neonatal seizure detection system: a clinician’s perspective. Clin Neurophysiol. 2011;122:1490–1499. - PubMed
-
- Clancy R.R., Legido A. The exact ictal and interictal duration of electroencephalographic neonatal seizures. Epilepsia. 1987;28:537–541. - PubMed
-
- Deburchgraeve W., Cherian P.J., De Vos M., Swarte R.M., Blok J.H., Visser G.H. Automated neonatal seizure detection mimicking a human observer reading EEG. Clin Neurophysiol. 2008;119:2447–2454. - PubMed
-
- Glass H.C., Bonifacio S.L., Shimotake T., Ferriero D.M. Neurocritical care for neonates. Curr Treat Options Neurol. 2011;13:574–589. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

