Anaemia in kidney disease: harnessing hypoxia responses for therapy
- PMID: 26055355
- PMCID: PMC4497972
- DOI: 10.1038/nrneph.2015.82
Anaemia in kidney disease: harnessing hypoxia responses for therapy
Abstract
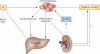
Improved understanding of the oxygen-dependent regulation of erythropoiesis has provided new insights into the pathogenesis of anaemia associated with renal failure and has led to the development of novel therapeutic agents for its treatment. Hypoxia-inducible factor (HIF)-2 is a key regulator of erythropoiesis and iron metabolism. HIF-2 is activated by hypoxic conditions and controls the production of erythropoietin by renal peritubular interstitial fibroblast-like cells and hepatocytes. In anaemia associated with renal disease, erythropoiesis is suppressed due to inadequate erythropoietin production in the kidney, inflammation and iron deficiency; however, pharmacologic agents that activate the HIF axis could provide a physiologic approach to the treatment of renal anaemia by mimicking hypoxia responses that coordinate erythropoiesis with iron metabolism. This Review discusses the functional inter-relationships between erythropoietin, iron and inflammatory mediators under physiologic conditions and in relation to the pathogenesis of renal anaemia, as well as recent insights into the molecular and cellular basis of erythropoietin production in the kidney. It furthermore provides a detailed overview of current clinical experience with pharmacologic activators of HIF signalling as a novel comprehensive and physiologic approach to the treatment of anaemia.
Figures

References
-
- Gregory CJ, Eaves AC. Human marrow cells capable of erythropoietic differentiation in vitro: definition of three erythroid colony responses. Blood. 1977;49:855–864. - PubMed
-
- Nagata S. Autoimmune diseases caused by defects in clearing dead cells and nuclei expelled from erythroid precursors. Immunol. Rev. 2007;220:237–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

