Treatment satisfaction and quality-of-life between type 2 diabetes patients initiating long- vs. intermediate-acting basal insulin therapy in combination with oral hypoglycemic agents--a randomized, prospective, crossover, open clinical trial
- PMID: 26055391
- PMCID: PMC4459660
- DOI: 10.1186/s12955-015-0279-4
Treatment satisfaction and quality-of-life between type 2 diabetes patients initiating long- vs. intermediate-acting basal insulin therapy in combination with oral hypoglycemic agents--a randomized, prospective, crossover, open clinical trial
Abstract
Background: Pharmacological and clinical differences between insulin glargine and NPH insulin may translate into differences in patient reported outcomes, but existing data are equivocal.
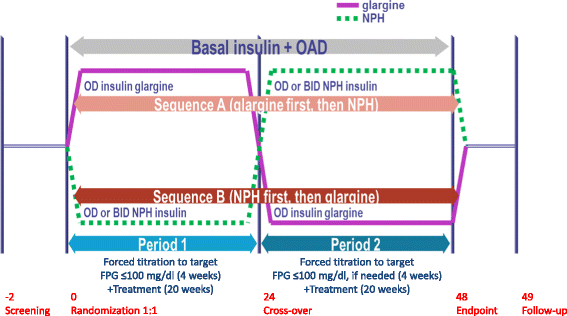
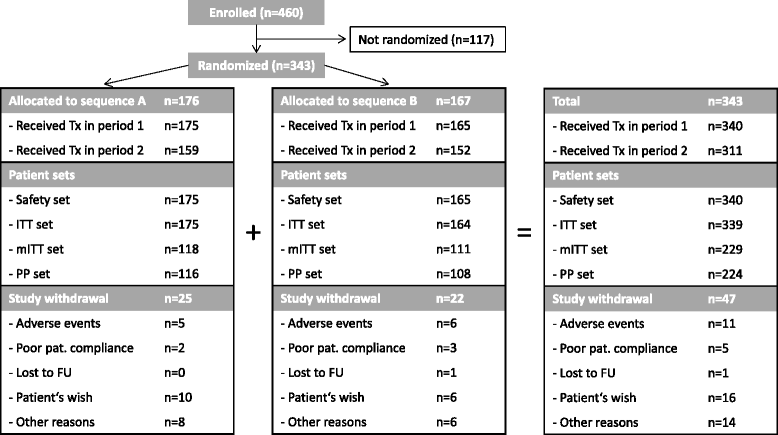
Methods: In this 48-week, open-label, randomized, multi-center, crossover phase IV trial, insulin naïve type 2 diabetes patients with blood glucose not at target on oral hypoglycemic agents had basal insulin added to their treatment regimen. A total of 343 patients were randomized to either receive insulin glargine (n = 176; sequence A) or neutral protamine Hagedorn (NPH) insulin (n = 167; sequence B) in period 1 (weeks 1-24) and vice versa in period 2 (weeks 25-48). The primary objective was to assess patient reported outcomes using a composite Diabetes Related Quality of Life (DRQoL) score based on an unweighted Insulin Treatment Experience Questionnaire (ITEQ) score, a Problem Areas in Diabetes (PAID) questionnaire score, and the mental health score in the Short Form (SF)-12® Health Survey, analyzed by analysis of covariance (ANCOVA).
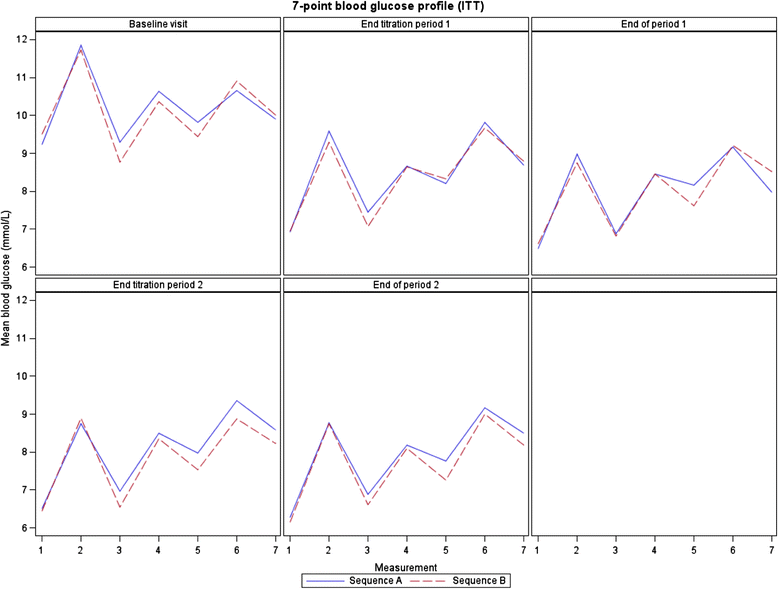
Results: Patients (mean age 62.3 ± 9.0; 39.5 % female) had a mean diabetes duration of 9.6 ± 5.9 years, a mean baseline HbA1c of 8.15 ± 0.72 %, and a mean fasting blood glucose (FBG) level of 9.37 ± 2.19 mmol/L. A total of 229 patients were available for primary endpoint evaluation (modified intention to treat population). Combining all data from both periods for each insulin treatment, on a 0-100 scale, the mean DRQoL score was 69.6 (±9.04) with insulin glargine and 70.0 (±9.40) with NPH insulin. Neither an effect of treatment with insulin glargine vs NPH insulin (p = 0.31) nor a period effect (p = 0.96), nor a sequence effect (p = 0.76) was observed using ANCOVA.
Conclusions: The results show that in a patient population with sub-optimal glycemic control at baseline, and a low target achievement rate together with a low rate of hypoglycemia, differences in the patient reported outcomes evaluated in this study were negligible between insulin glargine and NPH insulin.
Trial registration: Clinicaltrials.gov identifier: NCT00941369.
Figures
References
-
- Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ (Clinical research ed) 2000;321(7258):405–12. doi: 10.1136/bmj.321.7258.405. - DOI - PMC - PubMed
-
- Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. Jama. 1999;281(21):2005–12. doi: 10.1001/jama.281.21.2005. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

