Phosphorylation-dependent Regulation of Connecdenn/DENND1 Guanine Nucleotide Exchange Factors
- PMID: 26055712
- PMCID: PMC4505046
- DOI: 10.1074/jbc.M115.636712
Phosphorylation-dependent Regulation of Connecdenn/DENND1 Guanine Nucleotide Exchange Factors
Abstract
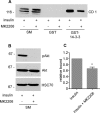
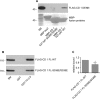
Connecdenn 1/2 are DENN (differentially expressed in normal and neoplastic cells) domain-bearing proteins that function as GEFs (guanine nucleotide exchange factors) for the small GTPase Rab35. Disruption of connecdenn/Rab35 function leads to defects in the recycling of multiple cargo proteins from endosomes with altered cell function, yet the regulation of connecdenn GEF activity is unexplored. We now demonstrate that connecdenn 1/2 are autoinhibited such that the purified, full-length proteins have significantly less Rab35 binding and GEF activity than the isolated DENN domain. Both proteins are phosphorylated with prominent phosphorylation sites between residues 500 and 600 of connecdenn 1. A large scale proteomics screen revealed that connecdenn 1 is phosphorylated at residues Ser-536 and Ser-538 in an Akt-dependent manner in response to insulin stimulation of adipocytes. Interestingly, we find that an Akt inhibitor reduces connecdenn 1 interaction with Rab35 after insulin treatment of adipocytes. Remarkably, a peptide flanking Ser-536/Ser-538 binds the DENN domain of connecdenn 1, whereas a phosphomimetic peptide does not. Moreover, connecdenn 1 interacts with 14-3-3 proteins, and this interaction is also disrupted by Akt inhibition and by mutation of Ser-536/Ser-538. We propose that Akt phosphorylation of connecdenn 1 downstream of insulin activation regulates connecdenn 1 function through an intramolecular interaction.
Keywords: DENN domain; Rab; Rab35; cell biology; connecdenn; endocytosis; endosome; guanine nucleotide exchange factor (GEF); intracellular trafficking; membrane trafficking.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Stenmark H. (2009) Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 10, 513–525 - PubMed
-
- Cherfils J., Zeghouf M. (2013) Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 93, 269–309 - PubMed
-
- Yohe M. E., Rossman K. L., Gardner O. S., Karnoub A. E., Snyder J. T., Gershburg S., Graves L. M., Der C. J., Sondek J. (2007) Auto-inhibition of the Dbl family protein Tim by an N-terminal helical motif. J. Biol. Chem. 282, 13813–13823 - PubMed
-
- Hussain N. K., Jenna S., Glogauer M., Quinn C. C., Wasiak S., Guipponi M., Antonarakis S. E., Kay B. K., Stossel T. P., Lamarche-Vane N., McPherson P. S. (2001) Endocytic protein intersectin-l regulates actin assembly via Cdc42 and N-WASP. Nat. Cell Biol. 3, 927–932 - PubMed
-
- Miyamoto Y., Torii T., Yamamori N., Ogata T., Tanoue A., Yamauchi J. (2013) Akt and PP2A reciprocally regulate the guanine nucleotide exchange factor Dock6 to control axon growth of sensory neurons. Sci. Signal. 6, ra15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

