Efficacy and safety of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional DMARDs in patients with RA at week 97 (SUMMACTA)
- PMID: 26056119
- PMCID: PMC4717437
- DOI: 10.1136/annrheumdis-2015-207281
Efficacy and safety of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional DMARDs in patients with RA at week 97 (SUMMACTA)
Abstract
Objectives: To evaluate the long-term efficacy and safety of subcutaneous (SC) tocilizumab (TCZ) versus intravenous (IV) TCZ, including switching formulations, in patients with rheumatoid arthritis (RA) and inadequate response to disease-modifying antirheumatic drugs (DMARDs).
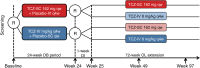
Methods: Patients (n=1262) were randomised 1:1 to receive TCZ-SC 162 mg weekly (qw)+placebo-IV every four weeks (q4w) or TCZ-IV 8 mg/kg q4w+placebo-SC qw in combination with DMARD(s). After a 24-week double-blind period, patients receiving TCZ-SC were re-randomised 11:1 to TCZ-SC (n=521) or TCZ-IV (TCZ-SC-IV, n=48), and patients receiving TCZ-IV were re-randomised 2:1 to TCZ-IV (n=372) or TCZ-SC (TCZ-IV-SC; n=186). Maintenance of clinical responses and safety through week 97 were assessed.
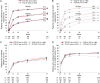
Results: The proportions of patients who achieved American College of Rheumatology (ACR)20/50/70 responses, Disease Activity Score in 28 joints remission and improvement from baseline in Health Assessment Questionnaire Disability Index ≥0.3 were sustained through week 97 and comparable across arms. TCZ-SC had a comparable safety profile to TCZ-IV through week 97, except that injection site reactions (ISRs) were more common with TCZ-SC. Safety profiles in patients who switched were similar to those in patients who received continuous TCZ-SC or TCZ-IV treatment. The proportion of patients who developed anti-TCZ antibodies remained low across treatment arms. No association between anti-TCZ antibody development and clinical response or adverse events was observed.
Conclusions: The long-term efficacy and safety of TCZ-SC was maintained and comparable to that of TCZ-IV, except for ISRs. Profiles in patients who switched formulations were comparable to those in patients who received TCZ-IV or TCZ-SC. TCZ-SC provides additional treatment options for patients with RA.
Trial registration number: NCT01194414.
Keywords: DMARDs (biologic); Rheumatoid Arthritis; Treatment.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures


References
-
- Genovese MC, McKay JD, Nasonov EL, et al. . Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum 2008;58:2968–80. 10.1002/art.23940 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

