Regulation by a chaperone improves substrate selectivity during cotranslational protein targeting
- PMID: 26056263
- PMCID: PMC4485088
- DOI: 10.1073/pnas.1422594112
Regulation by a chaperone improves substrate selectivity during cotranslational protein targeting
Abstract
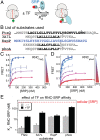
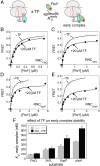
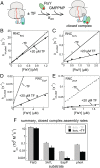
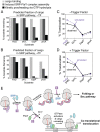
The ribosome exit site is a crowded environment where numerous factors contact nascent polypeptides to influence their folding, localization, and quality control. Timely and accurate selection of nascent polypeptides into the correct pathway is essential for proper protein biogenesis. To understand how this is accomplished, we probe the mechanism by which nascent polypeptides are accurately sorted between the major cotranslational chaperone trigger factor (TF) and the essential cotranslational targeting machinery, signal recognition particle (SRP). We show that TF regulates SRP function at three distinct stages, including binding of the translating ribosome, membrane targeting via recruitment of the SRP receptor, and rejection of ribosome-bound nascent polypeptides beyond a critical length. Together, these mechanisms enhance the specificity of substrate selection into both pathways. Our results reveal a multilayered mechanism of molecular interplay at the ribosome exit site, and provide a conceptual framework to understand how proteins are selected among distinct biogenesis machineries in this crowded environment.
Keywords: GTPases; protein biogenesis; ribosome; signal recognition particle; trigger factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ferbitz L, et al. Trigger factor in complex with the ribosome forms a molecular cradle for nascent proteins. Nature. 2004;431(7008):590–596. - PubMed
-
- Lakshmipathy SK, et al. Identification of nascent chain interaction sites on trigger factor. J Biol Chem. 2007;282(16):12186–12193. - PubMed
-
- Schlünzen F, et al. The binding mode of the trigger factor on the ribosome: Implications for protein folding and SRP interaction. Structure. 2005;13(11):1685–1694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

