Essential role of the cytochrome P450 CYP4F22 in the production of acylceramide, the key lipid for skin permeability barrier formation
- PMID: 26056268
- PMCID: PMC4485105
- DOI: 10.1073/pnas.1503491112
Essential role of the cytochrome P450 CYP4F22 in the production of acylceramide, the key lipid for skin permeability barrier formation
Abstract
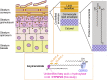
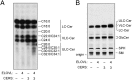
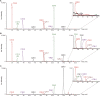
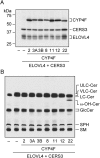
A skin permeability barrier is essential for terrestrial animals, and its impairment causes several cutaneous disorders such as ichthyosis and atopic dermatitis. Although acylceramide is an important lipid for the skin permeability barrier, details of its production have yet to be determined, leaving the molecular mechanism of skin permeability barrier formation unclear. Here we identified the cytochrome P450 gene CYP4F22 (cytochrome P450, family 4, subfamily F, polypeptide 22) as the long-sought fatty acid ω-hydroxylase gene required for acylceramide production. CYP4F22 has been identified as one of the autosomal recessive congenital ichthyosis-causative genes. Ichthyosis-mutant proteins exhibited reduced enzyme activity, indicating correlation between activity and pathology. Furthermore, lipid analysis of a patient with ichthyosis showed a drastic decrease in acylceramide production. We determined that CYP4F22 was a type I membrane protein that locates in the endoplasmic reticulum (ER), suggesting that the ω-hydroxylation occurs on the cytoplasmic side of the ER. The preferred substrate of the CYP4F22 was fatty acids with a carbon chain length of 28 or more (≥C28). In conclusion, our findings demonstrate that CYP4F22 is an ultra-long-chain fatty acid ω-hydroxylase responsible for acylceramide production and provide important insights into the molecular mechanisms of skin permeability barrier formation. Furthermore, based on the results obtained here, we proposed a detailed reaction series for acylceramide production.
Keywords: acylceramide; ceramide; lipid; skin; sphingolipid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Uchida Y, Holleran WM. Omega-O-acylceramide, a lipid essential for mammalian survival. J Dermatol Sci. 2008;51(2):77–87. - PubMed
-
- Mizutani Y, Mitsutake S, Tsuji K, Kihara A, Igarashi Y. Ceramide biosynthesis in keratinocyte and its role in skin function. Biochimie. 2009;91(6):784–790. - PubMed
-
- Breiden B, Sandhoff K. The role of sphingolipid metabolism in cutaneous permeability barrier formation. Biochim Biophys Acta. 2014;1841(3):441–452. - PubMed
-
- Kihara A. Very long-chain fatty acids: Elongation, physiology and related disorders. J Biochem. 2012;152(5):387–395. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

