Conserved SMP domains of the ERMES complex bind phospholipids and mediate tether assembly
- PMID: 26056272
- PMCID: PMC4485115
- DOI: 10.1073/pnas.1422363112
Conserved SMP domains of the ERMES complex bind phospholipids and mediate tether assembly
Abstract
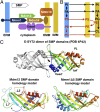
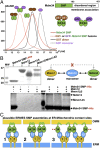
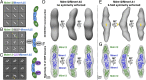
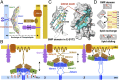
Membrane contact sites (MCS) between organelles are proposed as nexuses for the exchange of lipids, small molecules, and other signals crucial to cellular function and homeostasis. Various protein complexes, such as the endoplasmic reticulum-mitochondrial encounter structure (ERMES), function as dynamic molecular tethers between organelles. Here, we report the reconstitution and characterization of subcomplexes formed by the cytoplasm-exposed synaptotagmin-like mitochondrial lipid-binding protein (SMP) domains present in three of the five ERMES subunits--the soluble protein Mdm12, the endoplasmic reticulum (ER)-resident membrane protein Mmm1, and the mitochondrial membrane protein Mdm34. SMP domains are conserved lipid-binding domains found exclusively in proteins at MCS. We show that the SMP domains of Mdm12 and Mmm1 associate into a tight heterotetramer with equimolecular stoichiometry. Our 17-Å-resolution EM structure of the complex reveals an elongated crescent-shaped particle in which two Mdm12 subunits occupy symmetric but distal positions at the opposite ends of a central ER-anchored Mmm1 homodimer. Rigid body fitting of homology models of these SMP domains in the density maps reveals a distinctive extended tubular structure likely traversed by a hydrophobic tunnel. Furthermore, these two SMP domains bind phospholipids and display a strong preference for phosphatidylcholines, a class of phospholipids whose exchange between the ER and mitochondria is essential. Last, we show that the three SMP-containing ERMES subunits form a ternary complex in which Mdm12 bridges Mmm1 to Mdm34. Our findings highlight roles for SMP domains in ERMES assembly and phospholipid binding and suggest a structure-based mechanism for the facilitated transport of phospholipids between organelles.
Keywords: electron microscopy; interorganelle tether; membrane contact sites; membrane protein complex; phospholipid exchange.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Helle SC, et al. Organization and function of membrane contact sites. Biochim Biophys Acta. 2013;1833(11):2526–2541. - PubMed
-
- Elbaz Y, Schuldiner M. Staying in touch: The molecular era of organelle contact sites. Trends Biochem Sci. 2011;36(11):616–623. - PubMed
-
- Tatsuta T, Scharwey M, Langer T. Mitochondrial lipid trafficking. Trends Cell Biol. 2014;24(1):44–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1S10RR23057/RR/NCRR NIH HHS/United States
- S10 RR028893/RR/NCRR NIH HHS/United States
- UL1TR000124/TR/NCATS NIH HHS/United States
- P20 GM103418/GM/NIGMS NIH HHS/United States
- GM071940/GM/NIGMS NIH HHS/United States
- R01 GM103479/GM/NIGMS NIH HHS/United States
- UL1 TR000124/TR/NCATS NIH HHS/United States
- T32 GM007185/GM/NIGMS NIH HHS/United States
- R01 GM071940/GM/NIGMS NIH HHS/United States
- S10RR028893/RR/NCRR NIH HHS/United States
- R01GM103479/GM/NIGMS NIH HHS/United States
- S10 RR023057/RR/NCRR NIH HHS/United States

