High-resolution helix orientation in actin-bound myosin determined with a bifunctional spin label
- PMID: 26056276
- PMCID: PMC4491736
- DOI: 10.1073/pnas.1500625112
High-resolution helix orientation in actin-bound myosin determined with a bifunctional spin label
Abstract
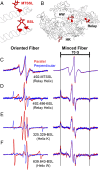
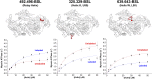
Using electron paramagnetic resonance (EPR) of a bifunctional spin label (BSL) bound stereospecifically to Dictyostelium myosin II, we determined with high resolution the orientation of individual structural elements in the catalytic domain while myosin is in complex with actin. BSL was attached to a pair of engineered cysteine side chains four residues apart on known α-helical segments, within a construct of the myosin catalytic domain that lacks other reactive cysteines. EPR spectra of BSL-myosin bound to actin in oriented muscle fibers showed sharp three-line spectra, indicating a well-defined orientation relative to the actin filament axis. Spectral analysis indicated that orientation of the spin label can be determined within <2.1° accuracy, and comparison with existing structural data in the absence of nucleotide indicates that helix orientation can also be determined with <4.2° accuracy. We used this approach to examine the crucial ADP release step in myosin's catalytic cycle and detected reversible rotations of two helices in actin-bound myosin in response to ADP binding and dissociation. One of these rotations has not been observed in myosin-only crystal structures.
Keywords: BSL; actomyosin; electron paramagnetic resonance; muscle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

