Caffeine acts through neuronal adenosine A2A receptors to prevent mood and memory dysfunction triggered by chronic stress
- PMID: 26056314
- PMCID: PMC4485143
- DOI: 10.1073/pnas.1423088112
Caffeine acts through neuronal adenosine A2A receptors to prevent mood and memory dysfunction triggered by chronic stress
Abstract
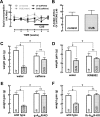
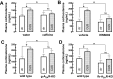
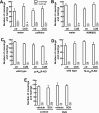
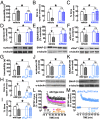
The consumption of caffeine (an adenosine receptor antagonist) correlates inversely with depression and memory deterioration, and adenosine A2A receptor (A2AR) antagonists emerge as candidate therapeutic targets because they control aberrant synaptic plasticity and afford neuroprotection. Therefore we tested the ability of A2AR to control the behavioral, electrophysiological, and neurochemical modifications caused by chronic unpredictable stress (CUS), which alters hippocampal circuits, dampens mood and memory performance, and enhances susceptibility to depression. CUS for 3 wk in adult mice induced anxiogenic and helpless-like behavior and decreased memory performance. These behavioral changes were accompanied by synaptic alterations, typified by a decrease in synaptic plasticity and a reduced density of synaptic proteins (synaptosomal-associated protein 25, syntaxin, and vesicular glutamate transporter type 1), together with an increased density of A2AR in glutamatergic terminals in the hippocampus. Except for anxiety, for which results were mixed, CUS-induced behavioral and synaptic alterations were prevented by (i) caffeine (1 g/L in the drinking water, starting 3 wk before and continued throughout CUS); (ii) the selective A2AR antagonist KW6002 (3 mg/kg, p.o.); (iii) global A2AR deletion; and (iv) selective A2AR deletion in forebrain neurons. Notably, A2AR blockade was not only prophylactic but also therapeutically efficacious, because a 3-wk treatment with the A2AR antagonist SCH58261 (0.1 mg/kg, i.p.) reversed the mood and synaptic dysfunction caused by CUS. These results herald a key role for synaptic A2AR in the control of chronic stress-induced modifications and suggest A2AR as candidate targets to alleviate the consequences of chronic stress on brain function.
Keywords: adenosine A2A receptor; caffeine; chronic stress; mood dysfunction; synaptic dysfunction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


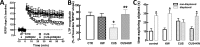


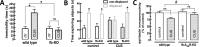

References
-
- de Kloet ER, Joëls M, Holsboer F. Stress and the brain: From adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–475. - PubMed
-
- McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87(3):873–904. - PubMed
-
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3(6):453–462. - PubMed
-
- Harris A, Ursin H, Murison R, Eriksen HR. Coffee, stress and cortisol in nursing staff. Psychoneuroendocrinology. 2007;32(4):322–330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

