Comparison of Single-Level and Multiple-Level Outcomes of Total Disc Arthroplasty: 24-Month Results
- PMID: 26056629
- PMCID: PMC4442630
- DOI: 10.14444/2014
Comparison of Single-Level and Multiple-Level Outcomes of Total Disc Arthroplasty: 24-Month Results
Abstract
Background: Low back pain is one of the most prevalent problems in industrialized countries, affecting as many as 80% of all adults at some time in their lives. Among the significant contributors to low back pain is degenerative disc disease (DDD). Although fusion has been well accepted for treatment of DDD, high rates of complications and stress to adjacent segments remain a concern. Lumbar total disc replacement (TDR) was developed with a goal of preserving motion and avoiding various fusion-related complications, but the relative merits of single vs. multiple level arthroplasty remain unclear.
Methods: This is a multi-center, single arm, prospective post-market registry of the M6-L, consisting of consecutive patients presenting with lumbar DDD who agreed to participate. This paper reports on those patients who have completed at least 24 months of followup to date. Clinical outcome measures include the Oswestry Disability Index (ODI) and back and leg Visual Analogue Scales (VAS). Radiographic analysis of disc angle and range of motion (ROM) was also performed.
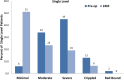
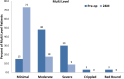
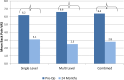
Results: Results for 83 patients comprising 121 implants in two cohorts (49 single level (SL), 34 multiple levels (ML)) are reported. Both cohorts experienced significant improvement at 24 months including significant decreases in ODI and VAS. Relative to SL procedures, ML procedures demonstrated either comparable results, or results that trended favorably towards the ML procedures. Index and global ROM at 24 months were not significantly different between the two cohorts, while the disc angles were larger in the SL cohort regardless of index level.
Conclusions: This is the first study to report clinical and radiographic outcomes of TDR with the M6-L in SL vs ML procedures with two years of followup. The results suggest initial device safety and effectiveness when used for the treatment of lumbar degenerative disc disease at one or more levels.
Keywords: Low Back Pain; lumbar disc disease; total disc replacement.
Figures





References
-
- Deyo RA. Low-back pain. Sci Am. Aug. 1998;279(2):48–53. - PubMed
-
- Canbulat N, Sasani M, Ataker Y, et al. A rehabilitation protocol for patients with lumbar degenerative disk disease treated with lumbar total disk replacement. Arch Phys Med Rehabil. 2011 Apr;92(4):670–676. - PubMed
-
- Blondel B, Tropiano P, Gaudart J, Marnay T. Clinical results of total lumbar disc replacement regarding various aetiologies of the disc degeneration: a study with a 2-year minimal follow-up. Spine (Phila Pa 1976). 2011 Mar 1;361(5):E313–319. - PubMed
-
- Blumenthal S, McAfee PC, Guyer RD, et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine. 2005 Jul 15;30(14):1565–1575. discussion E1387-1591. - PubMed
-
- Gornet MF, Burkus JK, Dryer RF, Peloza JH. Lumbar disc arthroplasty with Maverick disc versus stand-alone interbody fusion: a prospective, randomized, controlled, multicenter investigational device exemption trial. Spine (Phila Pa 1976). 2011 Dec 1;36(25):E1600–1611. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
