Treatment Extension of Pegylated Interferon Alpha and Ribavirin Does Not Improve SVR in Patients with Genotypes 2/3 without Rapid Virological Response (OPTEX Trial): A Prospective, Randomized, Two-Arm, Multicentre Phase IV Clinical Trial
- PMID: 26057627
- PMCID: PMC4461366
- DOI: 10.1371/journal.pone.0128069
Treatment Extension of Pegylated Interferon Alpha and Ribavirin Does Not Improve SVR in Patients with Genotypes 2/3 without Rapid Virological Response (OPTEX Trial): A Prospective, Randomized, Two-Arm, Multicentre Phase IV Clinical Trial
Abstract
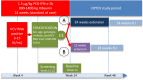
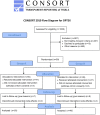
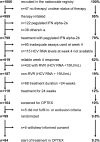
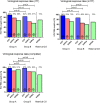
Although sofosbuvir has been approved for patients with genotypes 2/3 (G2/3), many parts of the world still consider pegylated Interferon alpha (P) and ribavirin (R) as standard of care for G2/3. Patients with rapid virological response (RVR) show response rates >80%. However, SVR (sustained virological response) in non-RVR patients is not satisfactory. Longer treatment duration may be required but evidence from prospective trials are lacking. A total of 1006 chronic HCV genotype 2/3 patients treated with P/R were recruited into a German HepNet multicenter screening registry. Of those, only 226 patients were still HCV RNA positive at week 4 (non-RVR). Non-RVR patients with ongoing response after 24 weeks P-2b/R qualified for OPTEX, a randomized trial investigating treatment extension of additional 24 weeks (total 48 weeks, Group A) or additional 12 weeks (total 36 weeks, group B) of 1.5 μg/kg P-2b and 800-1400 mg R. Due to the low number of patients without RVR, the number of 150 anticipated study patients was not met and only 99 non-RVR patients (n=50 Group A, n=49 Group B) could be enrolled into the OPTEX trial. Baseline factors did not differ between groups. Sixteen patients had G2 and 83 patients G3. Based on the ITT (intention-to-treat) analysis, 68% [55%; 81%] in Group A and 57% [43%; 71%] in Group B achieved SVR (p= 0.31). The primary endpoint of better SVR rates in Group A compared to a historical control group (SVR 70%) was not met. In conclusion, approximately 23% of G2/3 patients did not achieve RVR in a real world setting. However, subsequent recruitment in a treatment-extension study was difficult. Prolonged therapy beyond 24 weeks did not result in higher SVR compared to a historical control group.
Trial registration: ClinicalTrials.gov NCT00803309.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

