Engineered Nanostructures of Haptens Lead to Unexpected Formation of Membrane Nanotubes Connecting Rat Basophilic Leukemia Cells
- PMID: 26057701
- PMCID: PMC4758354
- DOI: 10.1021/acsnano.5b02270
Engineered Nanostructures of Haptens Lead to Unexpected Formation of Membrane Nanotubes Connecting Rat Basophilic Leukemia Cells
Abstract
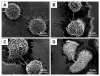
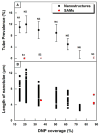
A recent finding reports that co-stimulation of the high-affinity immunoglobulin E (IgE) receptor (FcεRI) and the chemokine receptor 1 (CCR1) triggered formation of membrane nanotubes among bone-marrow-derived mast cells. The co-stimulation was attained using corresponding ligands: IgE binding antigen and macrophage inflammatory protein 1α (MIP1 α), respectively. However, this approach failed to trigger formation of nanotubes among rat basophilic leukemia (RBL) cells due to the lack of CCR1 on the cell surface (Int. Immunol. 2010, 22 (2), 113-128). RBL cells are frequently used as a model for mast cells and are best known for antibody-mediated activation via FcεRI. This work reports the successful formation of membrane nanotubes among RBLs using only one stimulus, a hapten of 2,4-dinitrophenyl (DNP) molecules, which are presented as nanostructures with our designed spatial arrangements. This observation underlines the significance of the local presentation of ligands in the context of impacting the cellular signaling cascades. In the case of RBL, certain DNP nanostructures suppress antigen-induced degranulation and facilitate the rearrangement of the cytoskeleton to form nanotubes. These results demonstrate an important scientific concept; engineered nanostructures enable cellular signaling cascades, where current technologies encounter great difficulties. More importantly, nanotechnology offers a new platform to selectively activate and/or inhibit desired cellular signaling cascades.
Keywords: atomic force microscopy (AFM); haptens; mast cells; membrane nanotubes; particle lithography; rat basophilic leukemia (RBL) cells; scanning electron microscopy (SEM).
Conflict of interest statement
Figures





References
-
- Gerdes HH, Bukoreshtliev NV, Barroso JFV. Tunneling Nanotubes: A New Route for the Exchange of Components between Animal Cells. FEBS Lett. 2007;581:2194–2201. - PubMed
-
- Onfelt B, Nedvetzki S, Yanagi K, Davis DM. Cutting Edge: Membrane Nanotubes Connect Immune Cells. J Immunol. 2004;173:1511–1513. - PubMed
-
- Watkins SC, Salter RD. Functional Connectivity between Immune Cells Mediated by Tunneling Nanotubules. Immunity. 2005;23:309–318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

