Families of microRNAs Expressed in Clusters Regulate Cell Signaling in Cervical Cancer
- PMID: 26057746
- PMCID: PMC4490472
- DOI: 10.3390/ijms160612773
Families of microRNAs Expressed in Clusters Regulate Cell Signaling in Cervical Cancer
Abstract
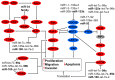
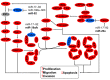
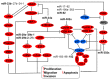
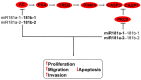
Tumor cells have developed advantages to acquire hallmarks of cancer like apoptosis resistance, increased proliferation, migration, and invasion through cell signaling pathway misregulation. The sequential activation of genes in a pathway is regulated by miRNAs. Loss or gain of miRNA expression could activate or repress a particular cell axis. It is well known that aberrant miRNA expression is well recognized as an important step in the development of cancer. Individual miRNA expression is reported without considering that miRNAs are grouped in clusters and may have similar functions, such as the case of clusters with anti-oncomiRs (23b~27b~24-1, miR-29a~29b-1, miR-29b-2~29c, miR-99a~125b-2, miR-99b~125a, miR-100~125b-1, miR-199a-2~214, and miR-302s) or oncomiRs activity (miR-1-1~133a-2, miR-1-2~133a-1, miR-133b~206, miR-17~92, miR-106a~363, miR183~96~182, miR-181a-1~181b-1, and miR-181a-2~181b-2), which regulated mitogen-activated protein kinases (MAPK), phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), NOTCH, proteasome-culling rings, and apoptosis cell signaling. In this work we point out the pathways regulated by families of miRNAs grouped in 20 clusters involved in cervical cancer. Reviewing how miRNA families expressed in cluster-regulated cell path signaling will increase the knowledge of cervical cancer progression, providing important information for therapeutic, diagnostic, and prognostic methodology design.
Keywords: cell signaling pathways; cervical cancer; clusters; families of miRNAs.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

