HIF-1α Promotes Epithelial-Mesenchymal Transition and Metastasis through Direct Regulation of ZEB1 in Colorectal Cancer
- PMID: 26057751
- PMCID: PMC4461314
- DOI: 10.1371/journal.pone.0129603
HIF-1α Promotes Epithelial-Mesenchymal Transition and Metastasis through Direct Regulation of ZEB1 in Colorectal Cancer
Abstract
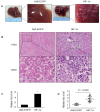
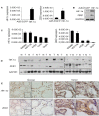
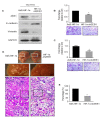
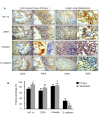
It is well recognized that hypoxia-inducible factor 1 alpha (HIF-1α) is involved in cancer metastasis, chemotherapy and poor prognosis. We previously found that deferoxamine, a hypoxia-mimetic agent, induces epithelial-mesenchymal transition (EMT) in colorectal cancer. Therefore, here we explored a new molecular mechanism for HIF-1α contributing to EMT and cancer metastasis through binding to ZEB1. In this study, we showed that overexpression of HIF-1α with adenovirus infection promoted EMT, cell invasion and migration in vitro and in vivo. On a molecular level, HIF-1α directly binding to the proximal promoter of ZEB1 via hypoxia response element (HRE) sites thus increasing the transactivity and expression of ZEB1. In addition, inhibition of ZEB1 was able to abrogate the HIF-1α-induced EMT and cell invasion. HIF-1α expression was highly correlated with the expression of ZEB1 in normal colorectal epithelium, primary and metastatic CRC tissues. Interestingly, both HIF-1α and ZEB1 were positively associated with Vimentin, an important mesenchymal marker of EMT, whereas negatively associated with E-cadherin expression. These findings suggest that HIF-1α enhances EMT and cancer metastasis by binding to ZEB1 promoter in CRC. HIF-1α and ZEB1 are both widely considered as tumor-initiating factors, but our results demonstrate that ZEB1 is a direct downstream of HIF-1α, suggesting a novel molecular mechanism for HIF-1α-inducing EMT and cancer metastasis.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

