A Functional Role for VEGFR1 Expressed in Peripheral Sensory Neurons in Cancer Pain
- PMID: 26058077
- PMCID: PMC4469373
- DOI: 10.1016/j.ccell.2015.04.017
A Functional Role for VEGFR1 Expressed in Peripheral Sensory Neurons in Cancer Pain
Erratum in
- Cancer Cell. 2015 Aug 10;28(2):270
Abstract
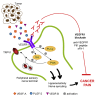
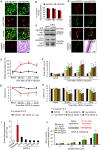
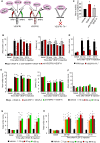
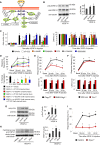
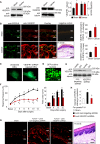
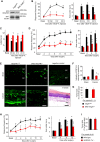
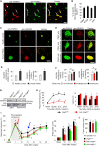
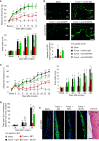
Cancer pain is a debilitating disorder and a primary determinant of the poor quality of life. Here, we report a non-vascular role for ligands of the Vascular Endothelial Growth Factor (VEGF) family in cancer pain. Tumor-derived VEGF-A, PLGF-2, and VEGF-B augment pain sensitivity through selective activation of VEGF receptor 1 (VEGFR1) expressed in sensory neurons in human cancer and mouse models. Sensory-neuron-specific genetic deletion/silencing or local or systemic blockade of VEGFR1 prevented tumor-induced nerve remodeling and attenuated cancer pain in diverse mouse models in vivo. These findings identify a therapeutic potential for VEGFR1-modifying drugs in cancer pain and suggest a palliative effect for VEGF/VEGFR1-targeting anti-angiogenic tumor therapies.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Agarwal N., Offermanns S., Kuner R. Conditional gene deletion in primary nociceptive neurons of trigeminal ganglia and dorsal root ganglia. Genesis. 2004;38:122–129. - PubMed
-
- Bae D.G., Kim T.D., Li G., Yoon W.H., Chae C.B. Anti-flt1 peptide, a vascular endothelial growth factor receptor 1-specific hexapeptide, inhibits tumor growth and metastasis. Clin. Cancer Res. 2005;11:2651–2661. - PubMed
-
- Bais C., Wu X., Yao J., Yang S., Crawford Y., McCutcheon K., Tan C., Kolumam G., Vernes J.M., Eastham-Anderson J. PlGF blockade does not inhibit angiogenesis during primary tumor growth. Cell. 2010;141:166–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

