Inhibition of the Mitochondrial Protease ClpP as a Therapeutic Strategy for Human Acute Myeloid Leukemia
- PMID: 26058080
- PMCID: PMC4461837
- DOI: 10.1016/j.ccell.2015.05.004
Inhibition of the Mitochondrial Protease ClpP as a Therapeutic Strategy for Human Acute Myeloid Leukemia
Abstract
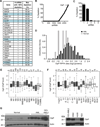
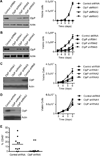
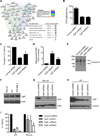
From an shRNA screen, we identified ClpP as a member of the mitochondrial proteome whose knockdown reduced the viability of K562 leukemic cells. Expression of this mitochondrial protease that has structural similarity to the cytoplasmic proteosome is increased in leukemic cells from approximately half of all patients with AML. Genetic or chemical inhibition of ClpP killed cells from both human AML cell lines and primary samples in which the cells showed elevated ClpP expression but did not affect their normal counterparts. Importantly, Clpp knockout mice were viable with normal hematopoiesis. Mechanistically, we found that ClpP interacts with mitochondrial respiratory chain proteins and metabolic enzymes, and knockdown of ClpP in leukemic cells inhibited oxidative phosphorylation and mitochondrial metabolism.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Antagonizing ClpP: A New Power Play in Targeted Therapy for AML.Cancer Cell. 2015 Jun 8;27(6):747-9. doi: 10.1016/j.ccell.2015.05.013. Cancer Cell. 2015. PMID: 26058072
References
-
- Dan S, Naito M, Tsuruo T. Selective induction of apoptosis in Philadelphia chromosome-positive chronic myelogenous leukemia cells by an inhibitor of BCR - ABL tyrosine kinase, CGP 57148. Cell Death Differ. 1998;5:710–715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

