Netrin-1 directs dendritic growth and connectivity of vertebrate central neurons in vivo
- PMID: 26058786
- PMCID: PMC4481067
- DOI: 10.1186/s13064-015-0041-y
Netrin-1 directs dendritic growth and connectivity of vertebrate central neurons in vivo
Abstract
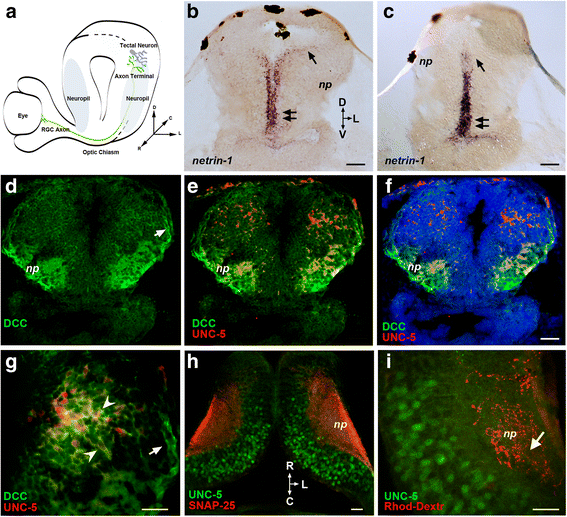
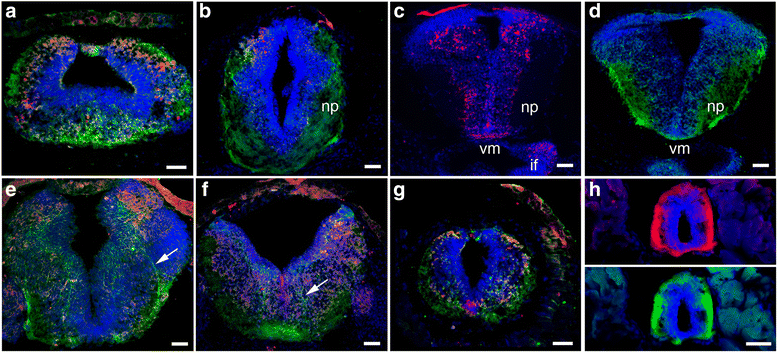
Background: Netrins are a family of extracellular proteins that function as chemotropic guidance cues for migrating cells and axons during neural development. In the visual system, netrin-1 has been shown to play a key role in retinal ganglion cell (RGC) axon growth and branching at the target, where presynaptic RGC axons form partnerships with the dendrites of tectal neurons. However, the signals that guide the connections between RGC axons and their postsynaptic partners are yet unknown. Here, we explored dynamic cellular mechanisms by which netrin-1 influences visual circuit formation, particularly those that impact postsynaptic neuronal morphology and connectivity during retinotectal wiring.
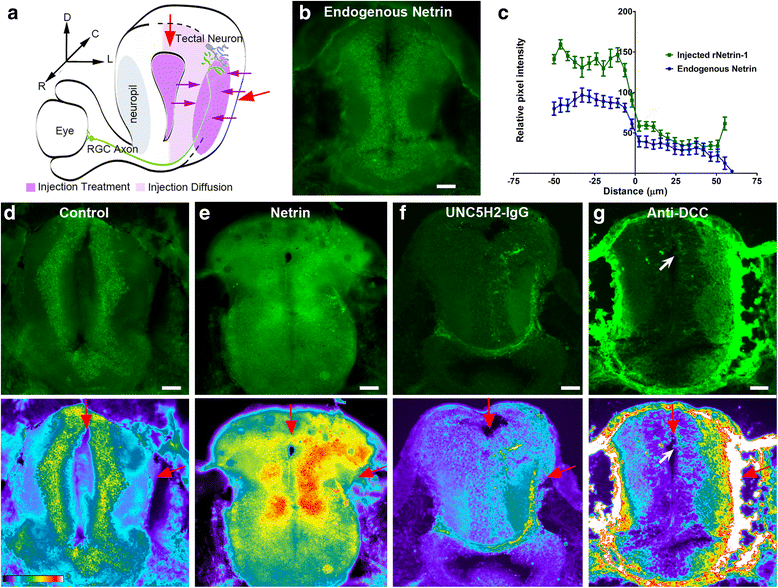
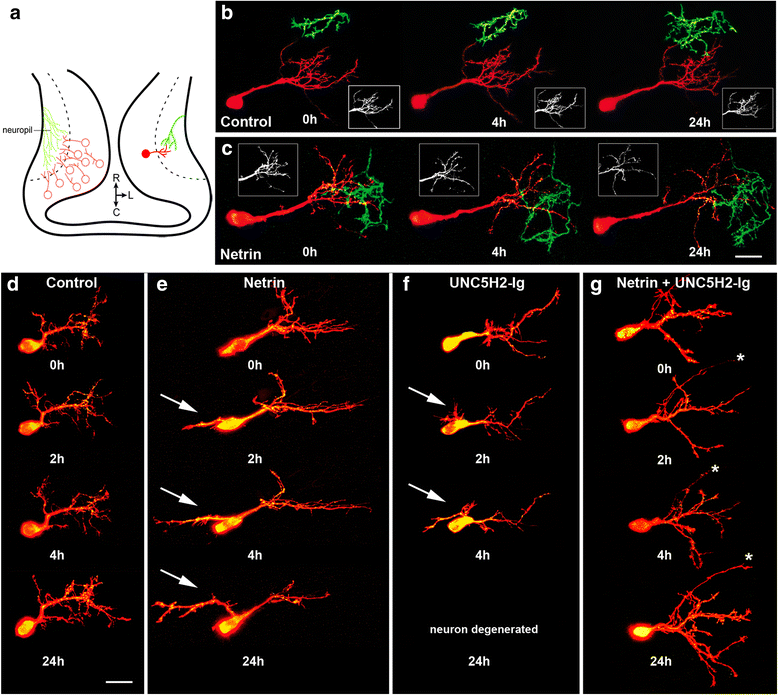
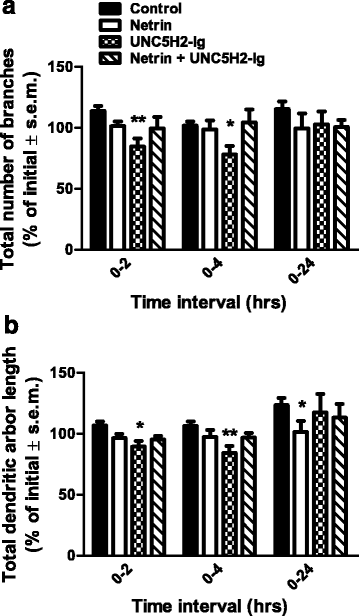
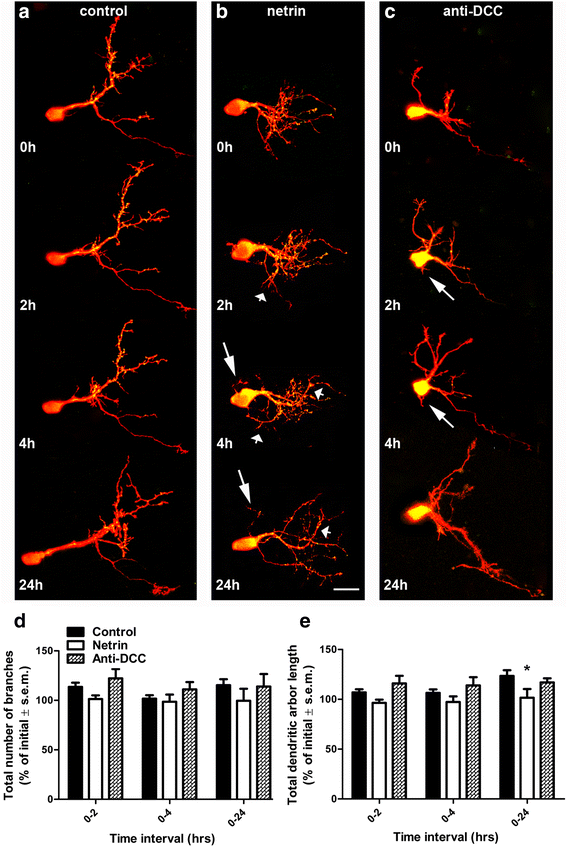
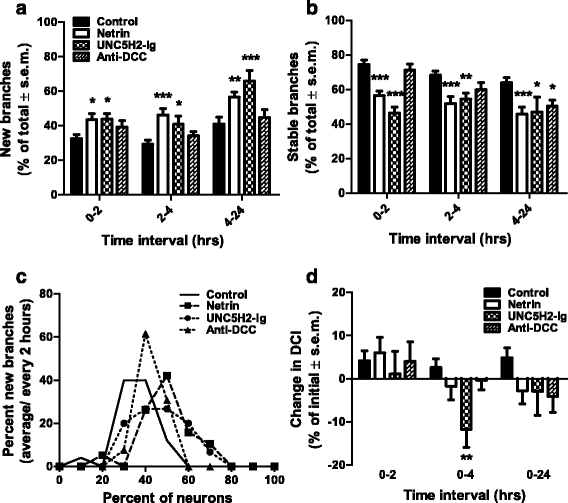
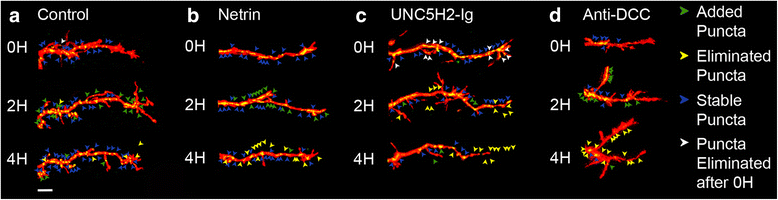
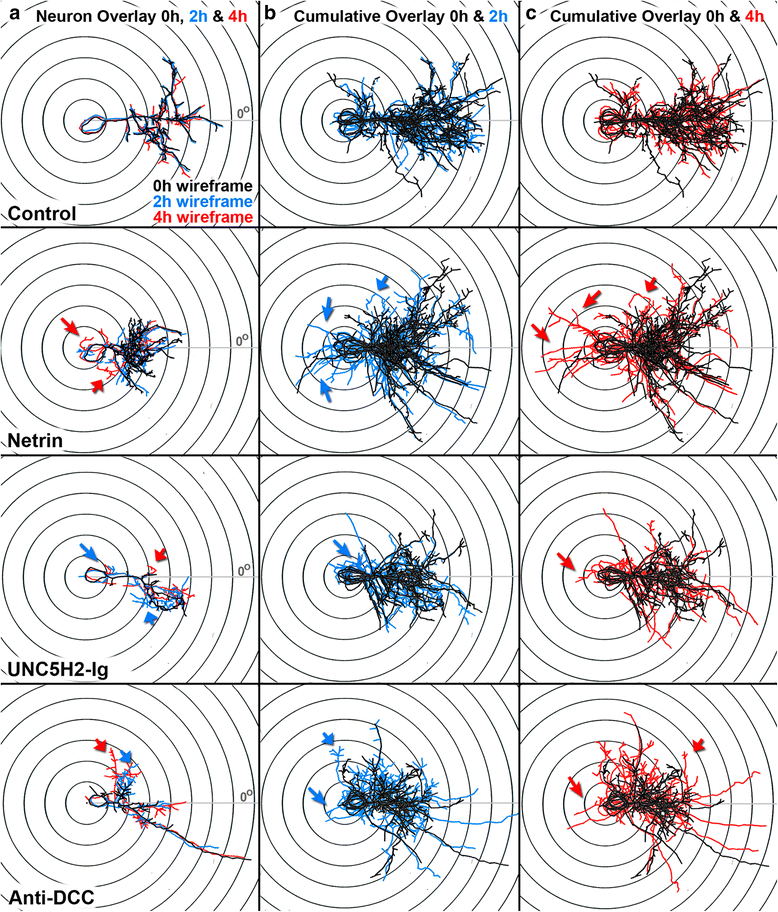
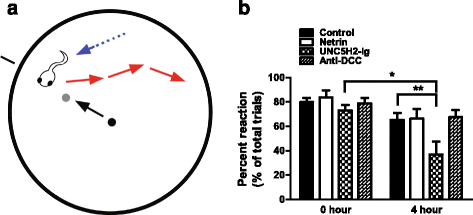
Results: Time-lapse in vivo imaging of individual Xenopus laevis optic tectal neurons co-expressing tdTomato and PSD95-GFP revealed rapid remodeling and reorganization of dendritic arbors following acute manipulations in netrin-1 levels. Effects of altered netrin signaling on developing dendritic arbors of tectal neurons were distinct from its effects on presynaptic RGC axons. Within 4 h of treatment, tectal injection of recombinant netrin-1 or sequestration of endogenous netrin with an UNC-5 receptor ectodomain induced significant changes in the directionality and orientation of dendrite growth and in the maintenance of already established dendrites, demonstrating that relative levels of netrin are important for these functions. In contrast, altering DCC-mediated netrin signaling with function-blocking antibodies induced postsynaptic specialization remodeling and changed growth directionality of already established dendrites. Reducing netrin signaling also decreased avoidance behavior in a visually guided task, suggesting that netrin is essential for emergent visual system function.
Conclusions: These in vivo findings together with the patterns of expression of netrin and its receptors reveal an important role for netrin in the early growth and guidance of vertebrate central neuron dendritic arbors. Collectively, our studies indicate that netrin shapes both pre- and postsynaptic arbor morphology directly and in multiple ways at stages critical for functional visual system development.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

