Telomere protein Rap1 is a charge resistant scaffolding protein in chromosomal bouquet formation
- PMID: 26058898
- PMCID: PMC4660835
- DOI: 10.1186/s12915-015-0149-x
Telomere protein Rap1 is a charge resistant scaffolding protein in chromosomal bouquet formation
Abstract
Background: Chromosomes reorganize in early meiotic prophase to form the so-called telomere bouquet. In fission yeast, telomeres localize to the nuclear periphery via interaction of the telomeric protein Rap1 with the membrane protein Bqt4. During meiotic prophase, the meiotic proteins Bqt1-2 bind Rap1 and tether to the spindle pole body to form the bouquet. Although it is known that this polarized chromosomal arrangement plays a crucial role in meiotic progression, the molecular mechanisms of telomere bouquet regulation are poorly understood.
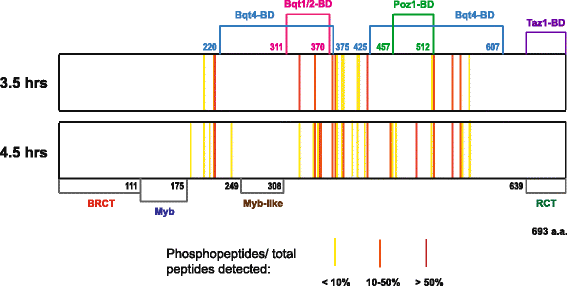
Results: Here, we detected high levels of Rap1 phospho-modification throughout meiotic prophase, and identified a maximum of 35 phosphorylation sites. Concomitant phosphomimetic mutation of the modification sites suggests that Rap1 hyper-phosphorylation does not directly regulate telomere bouquet formation or dissociation. Despite the negative charge conferred by its highly phosphorylated state, Rap1 maintains interactions with its binding partners. Interestingly, mutations that change the charge of negatively charged residues within the Bqt1-2 binding site of Rap1 abolished the affinity to the Bqt1-2 complex, suggesting that the intrinsic negative charge of Rap1 is crucial for telomere bouquet formation.
Conclusions: Whereas Rap1 hyper-phosphorylation observed in meiotic prophase does not have an apparent role in bouquet formation, the intrinsic negative charge of Rap1 is important for forming interactions with its binding partners. Thus, Rap1 is able to retain bouquet formation under heavily phosphorylated status.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

