Impact of Haemophilus influenzae type B (Hib) and viral influenza vaccinations in pregnancy for improving maternal, neonatal and infant health outcomes
- PMID: 26059051
- PMCID: PMC10786331
- DOI: 10.1002/14651858.CD009982.pub2
Impact of Haemophilus influenzae type B (Hib) and viral influenza vaccinations in pregnancy for improving maternal, neonatal and infant health outcomes
Abstract
Background: Infections during pregnancy confers increased risk of maternal and perinatal morbidity and mortality. However, the case for advocating Haemophilus influenzae type B (Hib) and viral Influenza vaccinations in pregnancy is still debatable.
Objectives: To assess the impact of Hib and viral Influenza vaccinations during pregnancy on maternal, neonatal and infant health outcomes compared to placebo/control.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (29 January 2015) and reference lists of retrieved studies.
Selection criteria: All randomised controlled clinical trials (including cluster-randomised trials) and quasi-randomised trials evaluating Hib or viral influenza vaccination during pregnancy compared with no vaccination or placebo.
Data collection and analysis: Two review authors independently assessed trials for inclusion, risk of bias and extracted data. Data were checked for accuracy.
Main results: Two trials were included this review. One (involving 213 women and 213 neonates) evaluated the impact of Hib vaccination during pregnancy and the other study (involving 2116 women and 2049 neonates) evaluated the impact of viral influenza vaccination during pregnancy. Overall, the HiB vaccination trial was judged to be at 'high risk of bias' due to inadequate randomisation while the other trial was judged to be at 'low risk of bias'. Hib vaccination during pregnancy versus placeboOne trial involving 213 women and 213 neonates evaluating the impact of Hib vaccination during pregnancy was included under this comparison. The study did not report on any of this review's prespecified primary outcomes (including mortality, respiratory tract infection and sepsis) or secondary outcomes (including adverse events) except preterm delivery. There was no clear difference between the Hib vaccination and placebo control groups in terms of preterm delivery (risk ratio (RR) 1.28, 95% confidence interval (CI) 0.12 to 13.86, one study, 213 participants), fetal distress (RR 1.23, 95% CI 0.67 to 2.26, one study, 213 infants), intubation (RR 1.03, 95% CI 0.55 to 1.95, one study, 213 infants) and neonatal jaundice (RR 1.01, 95% CI 0.52 to 1.97, one study, 213 infants). We could not grade the evidence for quality due to lack of outcome data. Viral influenza vaccination during pregnancy versus placeboOne trial involving 2116 women and 2049 infants evaluating the impact of trivalent inactivated influenza vaccine (IIV3) during pregnancy was included under this comparison.There was no clear difference between the viral influenza and placebo control group in terms of most of this review's primary outcomes: maternal death (RR 4.96, 95% CI 0.24 to 103.24, moderate quality evidence), infant death up to 175 days after birth (RR 0.71, 95% CI 0.37 to 1.37, moderate quality evidence), perinatal death (stillbirth and death in the first week of life) (RR 1.32, 95% CI 0.73 to 2.38, moderate quality evidence), influenza-like illness in women (RR 0.96, 95% CI 0.79 to 1.16) or their babies (RR 1.02, 95% CI 0.94 to 1.09), any respiratory illness in women (RR 0.97, 95% CI 0.91 to 1.04, high quality evidence) or their babies (RR 1.01, 95% CI 0.95 to 1.07, high quality evidence). There were also no clear differences between vaccination and placebo control groups in terms of maternal hospitalisation for any infection (RR 2.27, 95% CI 0.94 to 5.49; 2116 women, moderate quality evidence), and neonatal hospitalisation for sepsis (RR 1.60, 95% CI 0.73 to 3.50; 2049 infants, moderate quality evidence). However, viral influenza vaccination during pregnancy was associated with a reduction in reverse-transcriptase-polymerase-chain-reaction (RT-PCR) confirmed influenza among infants (RR 0.51, 95% CI 0.30 to 0.88, one study, 2049 infants) and women (RR 0.50, 95% CI 0.29 to 0.86, one study, 2116 women).In terms of this review's secondary outcomes, there were no clear differences in terms of the impact on pregnancy outcomes (miscarriage, preterm labour and stillbirth), hospitalisation for respiratory infection among women and infants. Similarly, there was no difference between the viral influenza vaccine and placebo control groups in terms of any adverse systemic reactions.
Authors' conclusions: There is limited evidence (from one small trial at a high risk of bias) on the effectiveness on Hib during pregnancy for improving maternal, neonatal and infant health outcomes.Evidence from one large high quality trial on the effectiveness of viral influenza vaccine during pregnancy suggests reduced RT-PCR confirmed influenza among women and their babies, suggesting the potential of this strategy for scale up but further evidence from varying contexts is required.Further trials for both Hib and viral influenza vaccines with appropriate study designs and suitable comparison groups are required. There are currently two 'ongoing' studies - these will be incorporated into the review in future updates.
Conflict of interest statement
None known.
Figures
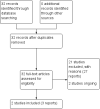
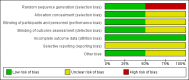






















Update of
- doi: 10.1002/14651858.CD009982
References
References to studies included in this review
Glezen 1992 {published data only}
-
- Glezen WP, Englund JA, Siber GR, Six HR, Turner C, Shriver D, et al. Maternal immunization with the capsular polysaccharide vaccine for haemophilus influenzae type b. Journal of Infectious Diseases 1992;165:134‐6. - PubMed
Madhi 2014 {published data only}
-
- Madhi SA. Vaccination of HIV‐uninfected pregnant women With trivalent influenza vaccine in the prevention of influenza illness during early infancy and in mothers: randomized controlled phase III trial evaluating safety, immunogenicity and efficacy. http://clinicaltrials.gov/ct2/show/record/NCT01306669 (accessed 29 June 2012).
-
- Madhi SA, Cutland CL, Kuwanda L, Weinberg A, Hugo A, Jones S, et al. Influenza vaccination of pregnant women and protection of their infants. New England Journal of Medicine 2014;371(10):918‐31. - PubMed
References to studies excluded from this review
Englund 1995 {published data only}
-
- Englund JA, Glezen WP, Turner C, Harvey J, Thompson C, Siber GR. Transplacental antibody transfer following maternal immunization with polysaccharide and conjugate haemophilus influenzae type b vaccines. Journal of Infectious Diseases 1995;171(1):99‐105. - PubMed
Henkle 2010 {published data only}
-
- Henkle E, Steinhoff MC, Omer SB, Roy OE, Arifeen SE, Raqib R, et al. Incidence of influenza infection in early infancy in Southern Asia. Pediatric Academic Societies' 2010 Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
Holmlund 2011 {published data only}
-
- Holmlund E, Nohynek H, Quiambao B, Ollgren J, Kayhty H. Mother‐infant vaccination with pneumococcal polysaccharide vaccine: Persistence of maternal antibodies and responses of infants to vaccination. Vaccine 2011;29(28):4565‐75. - PubMed
Jackson 2011 {published data only}
Madhi 2011a {published data only}
-
- Madhi SA. Trivalent influenza vaccine in HIV‐infected pregnant women and kinetics of transplacental anti‐influenza antibody transfer and persistence in young infants: a randomized controlled phase II trial evaluating safety and immunogenicity. http://clinicaltrials.gov/show/NCT01306682 (accessed 29 June 2012).
Moniz 2010 {published data only}
-
- Moniz M. Text messaging for preventative health during pregnancy; improving influenza vaccination rates in pregnancy: a randomized controlled trial of text messaging to increase vaccine uptake. http://clinicaltrials.gov/show/NCT01248520 (accessed 2012).
Mulholland 1996 {published data only}
-
- Mulholland K, Suara RO, Siber G, Roberton D, Jaffar S, N'Jie J. Maternal immunization with haemophilus influenzae type b polysaccharide‐tetanus protein conjugate vaccine in the Gambia. International Journal of Gynecology & Obstetrics 1996;55:89‐90. - PubMed
-
- Mulholland K, Suara RO, Siber G, Roberton D, Jaffar S, N'Jie J, et al. Maternal immunization with haemophilus influenzae type b polysaccharide‐tetanus protein conjugate vaccine in the Gambia. JAMA 1996;275(15):1182‐8. - PubMed
Munoz 2001 {published data only}
-
- Munoz FM, Englund JA, Cheesman CC, Maccato ML, Pinell PM, Nahm MH, et al. Maternal immunization with pneumococcal polysaccharide vaccine in the third trimester of gestation. Vaccine 2001;20(6):826‐37. - PubMed
NIAID 2009a {published data only}
-
- National Institute of Allergy and Infectious Diseases (NIAID). A phase II study in pregnant women to assess the safety and immunogenicity of an unadjuvanted Sanofi Pasteur H1N1 inactivated influenza vaccine administered at two dose levels. http://clinicaltrials.gov/ct2/show/record/NCT00963430 (accessed 2 July 2012).
NIAID 2009b {published data only}
-
- Johnson R. Influenza vaccine in pregnant women. http://www.clinicaltrials.gov/ct2/show/NCT00905125 (accessed 10 March 2011).
-
- National Institute of Allergy and Infectious Diseases (NIAID). A randomized, double‐blind trial on the safety and immunogenicity of inactivated trivalent influenza vaccine in pregnant women. http://clinicaltrials.gov/ct2/show/record/NCT00905125 (accessed 2 July 2012).
NIAID 2009c {published data only}
-
- National Institute of Allergy and Infectious Diseases (NIAID). A phase II study in pregnant women to assess the safety and immunogenicity of an unadjuvanted Novartis H1N1 inactivated influenza vaccine administered at two dose levels. http://clinicaltrials.gov/show/NCT00992719 (accessed 2 July 2012).
NIAID 2010 {published data only}
-
- National Institute of Allergy and Infectious Diseases (NIAID). 2010‐2011 trivalent influenza vaccine (TIV) in pregnant women. http://clinicaltrials.gov/show/NCT01173211 (accessed 10 March 2011).
Omer 2013 {published data only}
-
- Omer SB, Zaman K, Roy E, Arifeen SE, Raqib R, Noory L, et al. Combined effects of antenatal receipt of influenza vaccine by mothers and pneumococcal conjugate vaccine receipt by infants: Results from a randomized, blinded, controlled trial. Journal of Infectious Diseases 2013;207(7):1144‐7. - PubMed
Quiambao 2003 {published data only}
-
- Quiambao BP, Nohynek H, Kayhty H, Ollgren J, Gozum L, Gepanayao CP, et al. Maternal immunization with pneumococcal polysaccharide vaccine in the Philippines. Vaccine 2003;21(24):3451‐4. - PubMed
Quiambao 2007 {published data only}
-
- Quiambao BP, Nohynek HM, Kayhty H, Ollgren JP, Gozum LS, Gepanayao CP, et al. Immunogenicity and reactogenicity of 23‐valent pneumococcal polysaccharide vaccine among pregnant Filipino women and placental transfer of antibodies. Vaccine 2007;25(22):4470‐7. - PubMed
Schlaudecker 2013 {published data only}
Stockwell 2014 {published data only}
Tarrant 2013 {published data only}
-
- Tarrant M. A randomized controlled trial of a brief educational intervention to increase uptake of influenza vaccine among pregnant women. http://clinicaltrials.gov/ct2/show/NCT01772901 (accessed 21 May 2013).
Wong 2014 {published data only}
Wootten 2010 {published data only}
-
- Wootten SH. Opting in vs opting out: impact on influenza vaccination in pregnant women. http://clinicaltrials.gov/ct2/show/record/ 2012.
Zaman 2008 {published data only}
-
- Steinhoff MC, Omer SB, Roy E, Arifeen SE, Raqib R, Altaye M, et al. Influenza immunization in pregnancy and birth weights: observations from a randomised controlled trial. Pediatric Academic Societies' 2010 Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
-
- Steinhoff MC, Schlaudecker EP, Omer SB, Roy E, Altaye M, Zaman K. Antibody persistence in young adults one year after pneumococcal immunization in pregnancy. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 April 30‐May 3; Denver, Colorado, USA. 2011.
-
- Steinhoff MC, Schlaudecker EP, Omer SB, Roy E, Zaman K. Influenza IgA antibody in human milk: a randomised trial of maternal influenza immunization. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 April 30‐May 3; Denver, Colorado, USA. 2011.
-
- Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, et al. Effectiveness of maternal influenza immunization in mothers and infants. New England Journal of Medicine 2008;359(15):1555‐64. - PubMed
References to ongoing studies
Garcia 2012 {published data only}
-
- Garcia LG. Clinical trial to evaluate the immunogenicity and safety of the 2011‐2012 vaccine against seasonal influenza on pregnant women. http://clinicaltrials.gov/ct2/show/record/NCT01577316 (accessed 29 June 2012).
Tapia 2012 {published data only}
-
- Tapia MD. Prospective, randomized, controlled, observer‐blind trial to measure the efficacy, safety and immunogenicity of trivalent inactivated influenza vaccine and the safety and immunogenicity of quadrivalent meningococcal polysaccharide diphtheria conjugate vaccine in pregnant Malian women and their infants up to 6 months of age. http://clinicaltrials.gov/show/NCT01430689 (accessed 29 June 2012).
Additional references
Acs 2005
-
- Acs N, Banhidy F, Puho E, Czeizel AE. Maternal influenza during pregnancy and risk of congenital abnormalities in offspring. Birth Defects Research. Part A, Clinical and Molecular Teratology 2005;73:989‐96. - PubMed
Amstey 1985
-
- Amstey MS, Insel R, Munoz J, Pichichero M. Fetal‐neonatal passive immunization against Hemophilus influenzae, type b. American Journal of Obstetrics and Gynecology 1985;153(6):607‐11. - PubMed
Anderson 1977
-
- Anderson P, Smith DH, Ingram DL, Wilkins J, Wehrle F, Howie VM. Antibody to polyribophosphate of Haemophilus influenzae type b in infants and children: effect of immunization with polyribophosphate. Journal of Infectious Diseases 1977;136:S57‐S62. - PubMed
Black 2004
-
- Black SB, Shinefield HR, France EK, Fireman BH, Platt ST, Shay D, et al. Effectiveness of influenza vaccine during pregnancy in preventing hospitalizations and outpatient visits for respiratory illness in pregnant women and their infants. American Journal of Perinatology 2004;21(6):333‐9. - PubMed
Bridges 2013
-
- Bridges CB, Woods MDL, Coyne‐Beasley T. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedule for adults aged 19 years and older‐United States, 2013. Morbidity and Mortality Weekly Report. Surveillance Summaries : MMWR 2013;62(Suppl 1):9‐19. - PubMed
Chaithongwongwatthana 2012
Deinard 1981
-
- Deinard AS, Ogburn P Jr. A/NJ/8/76 influenza vaccination program: effects on maternal health and pregnancy outcome. American Journal of Obstetrics and Gynecology 1981;140:240–5. - PubMed
Englund 1993
-
- Englund JA, Mbawuike IN, Hammill H, Holleman MC, Baxter BD, Glezen WP. Maternal immunization with influenza or tetanus toxoid vaccine for passive antibody protection in young infants. Journal of Infectious Diseases 1993;168(3):647‐56. - PubMed
France 2006
-
- France EK, Smith‐Ray R, McClure D, Hambidge S, Xu S, Yamasaki K, et al. Impact of maternal influenza vaccination during pregnancy on the incidence of acute respiratory illness visits among infants. Archives of Pediatrics and Adolescent Medicine 2006;160(12):1277‐83. - PubMed
Gilbert‐Barness 2010
-
- Gilbert‐Barness E. Teratogenic causes of malformations. Annals of Clinical & Laboratory Science 2010;40:99‐114. - PubMed
Glezen 1997
-
- Glezen WP, Taber LH, Frank AL, Gruber WC, Piedra PA. Influenza virus infections in infants. Pediatric Infectious Disease Journal 1997;16(11):1065‐8. - PubMed
GRADE 2014 [Computer program]
-
- McMaster University. GRADEpro [Computer program on www.gradepro.org]. Version 2015. McMaster University, 2014.
Hartert 2003
-
- Hartert TV, Neuzil KM, Shintani AK, Mitchel EF Jr, Snowden MS, Wood LB, et al. Maternal morbidity and perinatal outcomes among pregnant women with respiratory hospitalizations during influenza season. American Journal of Obstetrics and Gynecology 2003;189(6):1705‐12. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Izurieta 2000
-
- Izurieta HS, Thompson WW, Kramarz P, Shay DK, Davis RL, DeStefano F, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. New England Journal of Medicine 2000;342(4):232‐9. - PubMed
Lindsay 2006
-
- Lindsay L, Jackson LA, Savitz DA, Weber DJ, Koch GG, Kong L, et al. Community influenza activity and risk of acute influenza‐like illness episodes among healthy unvaccinated pregnant and postpartum women. American Journal of Epidemiology 2006;163(9):838‐48. - PubMed
Mak 2008
-
- Mak TK, Mangtani P, Leese J, Watson JM, Pfeifer D. Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infectious Diseases 2008;8(1):44‐52. - PubMed
Munoz 2003
-
- Munoz FM, Greisinger AJ, Wehmanen OA, Mouzoon ME, Hoyle JC, Smith FA, et al. Influenza virus infection in infancy and early childhood. Paediatric Respiratory Reviews 2003;4(2):99‐104. - PubMed
Munoz 2005
-
- Munoz FM, Greisinger AJ, Wehmanen OA, Mouzoon ME, Hoyle JC, Smith FA, et al. Safety of influenza vaccination during pregnancy. American Journal of Obstetrics and Gynecology 2005;192(4):1098‐106. - PubMed
Neuzil 1998
-
- Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. American Journal of Epidemiology 1998;148(11):1094‐102. - PubMed
Puck 1980
-
- Puck JM, Glezen WP, Frank AL, Six HR. Protection of infants from infection with influenza A virus by transplacentally acquired antibody. Journal of Infectious Diseases 1980;142(6):844‐9. - PubMed
Reuman 1987
-
- Reuman PD, Ayoub EM, Small PA. Effect of passive maternal antibody on influenza illness in children: a prospective study of influenza A in mother‐infant pairs. Pediatric Infectious Disease Journal 1987;6(4):398‐403. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rudan 2004
Rudan 2008
Salam 2012
Santosham 2001
-
- Santosham M, Englund JA, McInnes P, Croll J, Thompson CM, Croll L, et al. Safety and antibody persistence following Haemophilus influenzae type b conjugate or pneumococcal polysaccharide vaccines given before pregnancy in women of childbearing age and their infants. Pediatric Infectious Disease Journal 2001;20(10):931‐40. - PubMed
Schunemann 2009
-
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. - PubMed
Shi 2005
-
- Shi L, Tu N, Patterson PH. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. International Journal of Developmental Neuroscience 2005;23:299‐305. - PubMed
Sumaya 1979
-
- Sumaya CV, Gibbs RS. Immunization of pregnant women with influenza A/New Jersey/76 virus vaccine: reactogenicity and immunogenicity in mother and infant. Journal of Infectious Diseases 1979;140(2):141‐6. - PubMed
Walker 2013
WHO 2005
-
- WHO. Haemophilus influenzae type B (HiB). http://www.who.int/mediacentre/factsheets/fs294/en/index.html (accessed 2012) 2005.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

