The semantic anatomical network: Evidence from healthy and brain-damaged patient populations
- PMID: 26059098
- PMCID: PMC6869673
- DOI: 10.1002/hbm.22858
The semantic anatomical network: Evidence from healthy and brain-damaged patient populations
Abstract
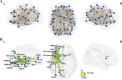
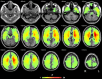
Semantic processing is central to cognition and is supported by widely distributed gray matter (GM) regions and white matter (WM) tracts. The exact manner in which GM regions are anatomically connected to process semantics remains unknown. We mapped the semantic anatomical network (connectome) by conducting diffusion imaging tractography in 48 healthy participants across 90 GM "nodes," and correlating the integrity of each obtained WM edge and semantic performance across 80 brain-damaged patients. Fifty-three WM edges were obtained whose lower integrity associated with semantic deficits and together with their linked GM nodes constitute a semantic WM network. Graph analyses of this network revealed three structurally segregated modules that point to distinct semantic processing components and identified network hubs and connectors that are central in the communication across the subnetworks. Together, our results provide an anatomical framework of human semantic network, advancing the understanding of the structural substrates supporting semantic processing.
Keywords: connectomics; diffusion tensor imaging; module; semantics; white-matter network.
© 2015 Wiley Periodicals, Inc.
Figures


Similar articles
-
White matter structural connectivity underlying semantic processing: evidence from brain damaged patients.Brain. 2013 Oct;136(Pt 10):2952-65. doi: 10.1093/brain/awt205. Epub 2013 Aug 23. Brain. 2013. PMID: 23975453
-
Unraveling the relationship between regional gray matter atrophy and pathology in connected white matter tracts in long-standing multiple sclerosis.Hum Brain Mapp. 2015 May;36(5):1796-807. doi: 10.1002/hbm.22738. Epub 2015 Jan 27. Hum Brain Mapp. 2015. PMID: 25627545 Free PMC article.
-
White matter pathway supporting phonological encoding in speech production: a multi-modal imaging study of brain damage patients.Brain Struct Funct. 2016 Jan;221(1):577-89. doi: 10.1007/s00429-014-0926-2. Epub 2014 Oct 31. Brain Struct Funct. 2016. PMID: 25359657
-
Clarifying Human White Matter.Annu Rev Neurosci. 2016 Jul 8;39:103-28. doi: 10.1146/annurev-neuro-070815-013815. Epub 2016 Apr 1. Annu Rev Neurosci. 2016. PMID: 27050319 Review.
-
The white matter architecture underlying semantic processing: A systematic review.Neuropsychologia. 2020 Jan;136:107182. doi: 10.1016/j.neuropsychologia.2019.107182. Epub 2019 Sep 27. Neuropsychologia. 2020. PMID: 31568774
Cited by
-
The Microstructural Plasticity of the Arcuate Fasciculus Undergirds Improved Speech in Noise Perception in Musicians.Cereb Cortex. 2021 Jul 29;31(9):3975-3985. doi: 10.1093/cercor/bhab063. Cereb Cortex. 2021. PMID: 34037726 Free PMC article.
-
Object knowledge representation in the human visual cortex requires a connection with the language system.PLoS Biol. 2025 May 20;23(5):e3003161. doi: 10.1371/journal.pbio.3003161. eCollection 2025 May. PLoS Biol. 2025. PMID: 40392802 Free PMC article.
-
Neural network bases of thematic semantic processing in language production.Cortex. 2022 Nov;156:126-143. doi: 10.1016/j.cortex.2022.08.007. Epub 2022 Sep 20. Cortex. 2022. PMID: 36244204 Free PMC article.
-
How the Brain Understands Spoken and Sung Sentences.Brain Sci. 2020 Jan 8;10(1):36. doi: 10.3390/brainsci10010036. Brain Sci. 2020. PMID: 31936356 Free PMC article.
-
Language in Behavioral Variant Frontotemporal Dementia: Another Stone to Be Turned in Latin America.Front Neurol. 2021 Aug 10;12:702770. doi: 10.3389/fneur.2021.702770. eCollection 2021. Front Neurol. 2021. PMID: 34447348 Free PMC article. Review.
References
-
- Acosta‐Cabronero J, Williams GB, Pengas G, Nestor PJ (2010): Absolute diffusivities define the landscape of white matter degeneration in alzheimer's disease. Brain 133(Pt 2):529–539. - PubMed
-
- Acosta‐Cabronero J, Patterson K, Fryer TD, Hodges JR, Pengas G, Williams GB, Nestor PJ (2011): Atrophy, hypometabolism and white matter abnormalities in semantic dementia tell a coherent story. Brain 134(Pt 7):2025–2035. - PubMed
-
- Aralasmak A, Ulmer JL, Kocak M, Salvan CV, Hillis AE, Yousem DM (2006): Association, commissural, and projection pathways and their functional deficit reported in literature. J Comput Assist Tomogr 30:695–715. - PubMed
-
- Assaf M, Calhoun VD, Kuzu CH, Kraut MA, Rivkin PR, Hart J Jr, Pearlson GD (2006): Neural correlates of the object‐recall process in semantic memory. Psychiatry Res 147:115–126. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

