Synthesis of phase-pure and monodisperse iron oxide nanoparticles by thermal decomposition
- PMID: 26059262
- PMCID: PMC5198837
- DOI: 10.1039/c5nr01651g
Synthesis of phase-pure and monodisperse iron oxide nanoparticles by thermal decomposition
Abstract
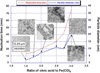
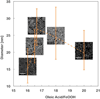
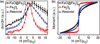
Superparamagnetic iron oxide nanoparticles (SPIONs) are used for a wide range of biomedical applications requiring precise control over their physical and magnetic properties, which are dependent on their size and crystallographic phase. Here we present a comprehensive template for the design and synthesis of iron oxide nanoparticles with control over size, size distribution, phase, and resulting magnetic properties. We investigate critical parameters for synthesis of monodisperse SPIONs by organic thermal decomposition. Three different, commonly used, iron containing precursors (iron oleate, iron pentacarbonyl, and iron oxyhydroxide) are evaluated under a variety of synthetic conditions. We compare the suitability of these three kinetically controlled synthesis protocols, which have in common the use of iron oleate as a starting precursor or reaction intermediate, for producing nanoparticles with specific size and magnetic properties. Monodisperse particles were produced over a tunable range of sizes from approximately 2-30 nm. Reaction parameters such as precursor concentration, addition of surfactant, temperature, ramp rate, and time were adjusted to kinetically control size and size-distribution, phase, and magnetic properties. In particular, large quantities of excess surfactant (up to 25 : 1 molar ratio) alter reaction kinetics and result in larger particles with uniform size; however, there is often a trade-off between large particles and a narrow size distribution. Iron oxide phase, in addition to nanoparticle size and shape, is critical for establishing magnetic properties such as differential susceptibility (dm/dH) and anisotropy. As an example, we show the importance of obtaining the required size and iron oxide phase for application to Magnetic Particle Imaging (MPI), and describe how phase purity can be controlled. These results provide much of the information necessary to determine which iron oxide synthesis protocol is best suited to a particular application.
Figures




References
-
- Weissleder R, et al. Superparamagnetic iron oxide: pharmacokinetics and toxicity. Am. J. Roentgenol. 1989;152:167–173. - PubMed
-
- Wang Y-XJ, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur. Radiol. 2001;11:2319–2331. - PubMed
-
- Lu M, Cohen MH, Rieves D, Pazdur R. FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am. J. Hematol. 2010 NA–NA. - PubMed
-
- Reimer P, Balzer T. Ferucarbotran (Resovist): a new clinically approved RES-specific contrast agent for contrast-enhanced MRI of the liver: properties, clinical development, and applications. Eur. Radiol. 2003;13:1266–1276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

