Prevalence and Perioperative Outcomes of Off-Label Total Hip and Knee Arthroplasty in the United States, 2000-2010
- PMID: 26059502
- PMCID: PMC4640948
- DOI: 10.1016/j.arth.2015.05.020
Prevalence and Perioperative Outcomes of Off-Label Total Hip and Knee Arthroplasty in the United States, 2000-2010
Abstract
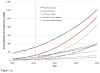
"Off-label use" refers to medical device utilization for purposes or subpopulations other than those approved by the United States Food and Drug Administration. The primary goal of this study was to determine the current epidemiology of off-label total hip and knee arthroplasties (THA and TKA, respectively) in the United States and to project further off-label use through 2040. Over the past decade, the prevalence of off-label THA and TKA was 30.4% and 37.0%, respectively, growing ~70% from 2000 to 2010. By 2040, the majority of THAs (86.1%) and TKAs (91.5%) could be off-label. The high prevalence of off-label arthroplasty and the dramatically shifting patient profile illustrated by these results highlight the need for continued medical device surveillance among on- and off label patients.
Keywords: complications; off-label; prevalence; projections; total hip arthroplasty; total knee arthroplasty.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

References
-
- U.S. Food and Drug Administration. U.S. Food and Drug Administration, Guidance for Institutional Review Boards and Clinical Investigators. “Off-Label” and Investigational Use of Marketed Drugs, Biologics and Medical Devices. 1998 http://www.fda.gov/RegulatoryInformation/Guidances/ucm126486.htm.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

